-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Ranking
Products & Resources
-
Study Abroad
Top Countries
Student Visas
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Exams
Colleges & Courses
Predictors
-
Learn
Online Courses
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Animation Courses
Colleges
Resources
-
Media, Mass Communication and Journalism
Exams
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


All Questions
The electron in the hydrogen atom undergoes transition from higher orbitals to orbital of radius 211.6 pm. This transition is associated with :
Option: 1 Lyman series
Option: 2 Balmer series
Option: 3 Paschen series
Option: 4 Brackett series
For hydrogen atom, the radius of n??????th orbit, r??????n = 52.9 pm ×n2
211.6 pm = 52.9 pm×n2
n??????2=4
n=2
Hence, the transition is from a higher orbit to the second orbit.
This corresponds to the Balmer series.
For the Balmer series in the spectrum of H atom, the correct statements among (I) to (IV) are: (1) As wavelength decreases, the lines in the series converge
(II) The integer n1 is equal to 2
(III) The lines of longest wavelength corresponds to n2 = 3
(IV) The ionization energy of hydrogen can be calculated from wavenumber of these lines
Option: 1
Option: 2
Option: 3
Option: 4
Line Spectrum of Hydrogen-like atoms
Where R is called Rydberg constant, R = 1.097 X 107, Z is the atomic number
n1= 1, 2, 3….
n2= n1+1, n1+2 ……
Lyman Series spectrum:
Where
n1= 1 and n2= 2, 3, 4....
This lies in the Ultraviolet region.
Balmer Series Spectrum:
Where n1= 2 and n2= 3, 4, 5, 6....
It lies in the visible region.
Paschen, Bracket and Pfund Series spectrums:
these lies in Infrared Region.
-
Since, so for wavelength to be longest or maximum the energy gap should be minimum. For the Balmer series, the transition for the longest wavelength is from n = 3 to n = 2. Thus clearly, n1 value for the Balmer series is 2. Further, as wavelength decreases, the lines in the series converge.
Therefore, Option(3) is correct.
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

The number of orbitals associated with quantum numbers
is:
Option: 1 25
Option: 2 11
Option: 3 15
Option: 4 50
We have:
n = 5, ms = +1/2
Thus, the values of l are from 0 to (n-1)
l = 0 to 4
Thus, values of l are 5s, 5p, 5d, 5f, and 5g
Now, the total number of orbitals
Therefore, Option(1) is correct.
View Full Answer(1)The radius of the second Bohr orbit, in terms of the Bohr radius, in
is :
Option: 1
Option: 2
Option: 3
Option: 4
As we have learnt,
Radius, velocity and the energy of nth Bohr orbital -
Bohr radius of nth orbit:
where Z is the atomic number
Velocity of electron in nth orbit:
where z is atomic number
Total energy of electron in nth orbit:
Where z is atomic number
-
.
Therefore, Option(2) is correct.
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
In the sixth period, the orbitals that are filled are :
Option: 1 6s, 4f, 5d, 6p
Option: 2 6s, 5d, 5f, 6p
Option: 3 6s, 5f, 6d, 6p
Option: 4 6s, 6p, 6d, 6f
Energy order of orbital’s according to Aufbau principle-

The order of orbitals filling is 6s, 4f, 5d, 6p.
Therefore, the correct option is (1).
View Full Answer(1)The difference between the radii of 3rd and 4th orbits of  is
is 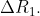 The difference between the radii of 3rd and 4th orbits of
The difference between the radii of 3rd and 4th orbits of  is
is 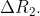 Ratio
Ratio 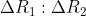 is :
is :
Option: 1 8 : 3
Option: 2 3 : 8
Option: 3 2 : 3
Option: 4 3 : 2
We know the formula,
The radius of nth Bohr's Orbit
Where
n = principal quantum number of orbit.
Z = Atomic number
Now,
The difference between the radii of 3rd and 4th orbits of is
The difference between the radii of 3rd and 4th orbits of is
Ratio is
Therefore, the correct option is (3).
View Full Answer(1)NEET 2024 Most scoring concepts
- Just Study 32% of the NEET syllabus and Score up to 100% marks
The correct statement about parobability density (except at a distance from nucleus) is :-
Option: 1 it can be zero for 1s orbital
Option: 2 It can never be zero for 2s orbital
Option: 3 It can be negative for 2p orbital
Option: 4 it can be zero for 3p orbital
Probability density can be zero for 3p orbital.
Therefore, Option(4) is correct.
View Full Answer(1)The region in the electromagnetic spectrum where the Balmer series lines appear is:
Option: 1 Visible
Option: 2 Microwave
Option: 3 Infrared
Option: 4 Ultraviolet
Balmer series give visible lines for H-atom.
As lines of Balamer series belongs to both UV as well visible region of EM spectrum. However most appropriate should be visible region.
Therefore, the correct option is (1).
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

The shortest wavelength of H atom in the Lyman series is 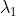 . The longest wavelength in the Balmer series He+ is:
. The longest wavelength in the Balmer series He+ is:
Option: 1 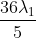
Option: 2 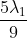
Option: 3 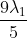
Option: 4 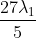
We know,
So, for
minimum(Shortest)
for f H atoms in the Lyman series n = 1 & for Transition must be form n =
to n = 1,
Z = 1
For longest wavelength for Balmer series n = 3 to n = 2 will have
for He+ , Z = 2
Therefore, the correct option is (3).
View Full Answer(1)Consider the hypothetical situation where the azimuthal quantum number, 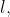 takes values
takes values  where
where 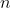 is the principal quantum number. Then, the element with atomic number :
is the principal quantum number. Then, the element with atomic number :
Option: 1 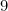 is the first alkali metal
is the first alkali metal
Option: 2 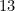 has a half-filled valence subshell
has a half-filled valence subshell
Option: 3 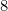 is the first noble gas
is the first noble gas
Option: 4 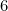 has a
has a 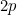 -valence subshell
-valence subshell
GIven l= 0 to (n + 1)
Now for n = 1,
l = 0, 1, 2
Orbitals :
1s , l =0
1p , l = 1
1d , l = 2
And for
n = 2
l = 0, 1, 2, 3
And for
n = 3
l = 0, 1, 2, 3,4
Now, in order to wire electronic configuration, we need to apply (n + l) rule.

Energy order : 1s < 1p < 2s < 1 d < 2p < 3s < 2d ...
1) 9 : 1s2 1p6 2s1 is the first alkali metal because after losing one electron, it will achieve the first noble gas configuration
2) 13 : 1s2 1p6 2s2 1d3 is not half filled. (Showing incorrect statement)
3) 8 : 1s2 1p6 is the first noble gas.
4) 6 : 1s2 1p4 has 1p valence subshell.
Therefore, Option 2 is correct.
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE


