-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Ranking
Products & Resources
-
Study Abroad
Top Countries
Student Visas
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Exams
Colleges & Courses
Predictors
-
Learn
Online Courses
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Animation Courses
Colleges
Resources
-
Media, Mass Communication and Journalism
Exams
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


All Questions
When photon of energy strikes the surface of a metal A, the ejected photoelectrons have maximum kinetic energy
and de-Broglie wavelength
. The maximum kinetic energy of photoelectrons liberated from another metal B by photon of energy
is
. If the de-Broglie wavelength of these photoelectrons
, then the work function(in eV) of metal B is:
Option: 1 4
Option: 2 1.5
Option: 3 3
Option: 4 2
As we know
where T=kinetic energy
So
Also
Using equation (1) and (2)
And using the Einstein equation
So the correct option is 1.
View Full Answer(1)A beam of electromagnetic radiation of intensity is comprised of wavelength
. It falls normally on a metal (work function
=2 eV) of surface area 1 cm2. If one in 103 photons ejects an electron, total number of electrons ejected in 1s is 10x (hc=1240 eVnm, 1 eV=1.6 x 10-19J), then x is :-
Option: 1 11
Option: 2 10
Option: 3 12
Option: 4 13
Energy in 1 second =
So the correct answer is option 1.
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

An electron of mass m and magnitude of charge initially at rest gets accelerated by a constant electric field E.The rate of change of de-broglie wavelength of this electron at time t is ? ( ignoring relativistic effects is) :
Option: 1
Option: 2
Option: 3
Option: 4
Hence the correct option is (1).
View Full Answer(1)An electron (mass m ) with initial velocity is in an electric field
. If
is initial de-Broglie wavelength of electron, its de-Broglie wavelength at time t is given by :
Option: 1
Option: 2
Option: 3
Option: 4
We know v=u+at and here
So, magnitude of velocity:-
Hence the correct option is (3).
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
Radiation with wavelength 6561 falls on a metal surface to produce photoelectrons. The electrons are made to enter a uniform magnetic field of
. If the radius of the largest circular path followed by the electrons is 10mm, the work function (in eV) of the metal is close to:
Option: 1 1.1
Option: 2 0.8
Option: 3 1.8
Option: 4 1.6
From photoelectric equation:-
Hence the option correct option is (1) .
View Full Answer(1)A particle moving with kinetic energy E has de Broglie wavelength . If energy
is added to its energy, the wavelength become
. Value of
, is:
Option: 1
Option: 2
Option: 3
Option: 4
Hence the option correct option is (3).
View Full Answer(1)NEET 2024 Most scoring concepts
- Just Study 32% of the NEET syllabus and Score up to 100% marks
An electron (of mass m) & a photon have the same energy E. Find the ratio of de Broglie wavelength of an electron and the wavelength of the photon (c = speed of light in vacuum).
Option: 1
Option: 2
Option: 3
Option: 4
Hence the correct option is (4).
View Full Answer(1)A particle of mass travels in a medium with a speed of
and a photon of a radiation of linear momentum
travels in vacuum. The wavelength of photon is _______ times the wavelength of the particle.
Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

In a photoelectric experiment, ultraviolet light of wavelength 280 nm is used with lithium cathode having work function 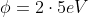 . If the wavelength of incident light is switched to 400 nm, find out the change in the stopping potential.
. If the wavelength of incident light is switched to 400 nm, find out the change in the stopping potential. 
Option: 1 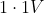
Option: 2 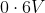
Option: 3 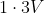
Option: 4 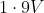
By Einstein's photoelectric eqn,
The correct option is (3)
The de-Broglie wavelength of a paraticle having kinetic energy E is 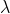 . How much extra energy must be given to this particle so that the de-Broglie wavelength reduces to 75% of the intial value?
. How much extra energy must be given to this particle so that the de-Broglie wavelength reduces to 75% of the intial value?
Option: 1 E
Option: 2 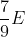
Option: 3 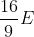
Option: 4 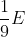
Extra energy is given =
The correct option is (2)
JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE


