-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Ranking
Products & Resources
-
Study Abroad
Top Countries
Student Visas
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Exams
Colleges & Courses
Predictors
-
Learn
Online Courses
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Animation Courses
Colleges
Resources
-
Media, Mass Communication and Journalism
Exams
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


All Questions
- #Engineering
- #Chemistry
- #Joint Entrance Examination Main
- #S - Block Elements (Alkali and Alkaline Earth Metals)
A metal (A) on heating in nitrogen gas gives compound B. B on treatment with gives a colourless gas which when passed through
solution gives a dark blue-violet coloured solution. A and B respectively, are :
Option: 1 and
Option: 2 and
Option: 3 and
Option: 4 and
Magnesium reacts with air to form its nitride. The nitride upon hydrolysis yields ammonia which gives a deep blue color solution with
View Full Answer(1)
- #Medical
- #Chemistry
- #Joint Entrance Examination Main
- #S - Block Elements (Alkali and Alkaline Earth Metals)
In the following reactions, products (A) and (B) respectively are: (hot and conc.)
(dry)
Option: 1
Option: 2
Option: 3
Option: 4
As we have learnt,
Chlorine reacts with and
and undergoes disproportionation. The products depend upon the concentration of the base and also the temperature.
The reactions are given as
(Hot and Conc.)
(Dry)
Therefore, Option(4) is correct.
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

- #Engineering
- #Chemistry
- #Joint Entrance Examination Main
- #S - Block Elements (Alkali and Alkaline Earth Metals)
Among the sulphate of alkaline earth metals the solubilities of and
in water, respectively, are -
Option: 1 high and poor
Option: 2 poor and poor
Option: 3 high and high
Option: 4 poor and high
and
are readily soluble in water. Thus, the solubility of both these sulphates is high in water.
Therefore, Option(3) is correct.
View Full Answer(1)- #Engineering
- #Chemistry
- #s- Block Elements ( Alkali and Alkaline earth metals )
- #Joint Entrance Examination Main
Match the following compounds (Column-I) with their uses (Column-II):
| S.No | Column-I | S.No | Column-II |
| (I) | (A) | casts of statues | |
| (II) | (B) | White wash | |
| (III) | (C) | antacid | |
| (IV) | (D) | washing soda preparation |
Option: 1 (I) - (D), (II) - (A), (III) - (C), (IV) -(B)
Option: 2 (I) - (B), (II) - (D), (III) - (A), (IV) -(C)
Option: 3 (I) - (B), (II) - (C), (III) - (D), (IV) -(A)
Option: 4 (I) - (C), (II) - (D), (III) - (B), (IV) -(A)
Compounds and their uses-

Therefore, the correct option is (2).
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
- #Engineering
- #Chemistry
- #s- Block Elements ( Alkali and Alkaline earth metals )
- #Joint Entrance Examination Main
One combination of Li, Na and K in excess of air, the major oxides formed, respectively are:
Option: 1 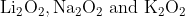
Option: 2 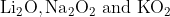
Option: 3 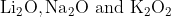
Option: 4 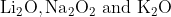
The formed oxides will be -
Therefore, the correct option is (2).
View Full Answer(1)- #Engineering
- #Chemistry
- #s- Block Elements ( Alkali and Alkaline earth metals )
- #Joint Entrance Examination Main
An alkaline earth metal 'M' readily forms water soluble sulphate and water insoluble hydroxide. Its oxide MO is very stable to heat and does not have rock-salt structure M is:
Option: 1 Sr
Option: 2 Ca
Option: 3 Mg
Option: 4 Be
M is "Be" alkaline earth metal.
Be readily forms water-soluble sulphate BaSO4
Be(OH)2 is water-insoluble hydroxide.
BeO is very stable to heat and does not have a rock-salt structure.
Therefore, the correct option is (4).
View Full Answer(1)NEET 2024 Most scoring concepts
- Just Study 32% of the NEET syllabus and Score up to 100% marks
- #Engineering
- #Chemistry
- #s- Block Elements ( Alkali and Alkaline earth metals )
- #Joint Entrance Examination Main
Among the statements (I–IV), the correct ones are:
(I) Be has smaller atomic radius compared to Mg.
(II)Be has higher ionization enthalpy than Al.
(III) Charge/radius ratio of Be is greater than that of Al.
(IV) Both Be and Al form mainly covalent compounds.
Option: 1 (I), (II) and (IV)
Option: 2 (I), (III) and (IV)
Option: 3 (II), (III) and (IV)
Option: 4 (I), (II) and (III)
(I) Be has a smaller atomic radius compared to Mg, in a group atomic radius increases.
(II) Be has higher ionization enthalpy than Al due to fully filled s-block orbital of Be.
(III) Charge/radius ratio of Be is greater than that of Al, due to Be being small.
(IV) Only Al form mainly covalent compounds, Be forms ionic compounds.
(I), (II), and (IV) are correct.
Therefore, Option 1 is correct.
View Full Answer(1)- #Engineering
- #Chemistry
- #s- Block Elements ( Alkali and Alkaline earth metals )
- #Joint Entrance Examination Main
Match List - I with List - II:
| List - I | List - II |
| (a) Ca(OCl)2 | (i) Antacid |
| (b) CaSO4.1/2 H2O | (ii) Cement |
| (c) CaO | (iii) Bleach |
| (d) CaCO3 | (iv) Plaster of Paris |
Option: 1 (a) - (iii), (b) -(iv), (c) - (ii), (d) -(i)
Option: 2 (a) - (iii), (b) -(ii), (c) - (i), (d) -(iv)
Option: 3 (a) - (i), (b) -(iv), (c) - (iii), (d) -(ii)
Option: 4 (a) - (iii), (b) -(ii), (c) - (iv), (d) -(i)
We know these facts.
Ca(OCl)2 is Bleach
CaSO4.1/2 H2O is Plaster of Paris.
CaCO3 is used as an antacid.
CaO is the major component of cement.
The correct matching : (a)-(iii), (b)-(iv), (c)-(ii), (d)-(i)
Therefore, the Correct option is (1)
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

- #Engineering
- #Chemistry
- #Joint Entrance Examination Main
- #S - Block Elements (Alkali and Alkaline Earth Metals)
The correct order of conductivity of ions in water is :
Option: 1 
Option: 2 
Option: 3 
Option: 4 
has lower hydrated radius so its electrical conductivity is higher.
Therefore, the correct option is (3)
View Full Answer(1)Given below are two statements : Statement I : Both CaCl2.6H2O and MgCl2.8H2O undergo dehydrationon heating. Statement II : BeO is amphoteric whereas the oxides of other elements in the same group are acidic. In the light of the above statements, choose the correct answer from the options given below :
Option: 1 Statement I is false but statement II is true
Option: 2 Both statement I and Statement II are false
Option: 3 Statement I is true but statement II is false
Option: 4 Both statement I and Statement II are true
The dehydration of hydrated chloride of calcium can be achieved. The corresponding hydrated chloride of magnesium on heating suffer hydrolysis.
MgO, CaO, SrO, BaO All are basic oxides.
So, Both Statement I and Statement II are false
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE


