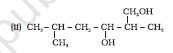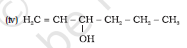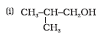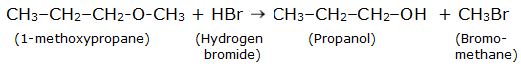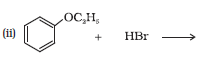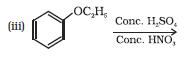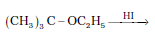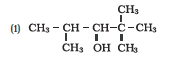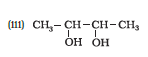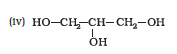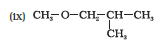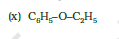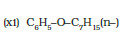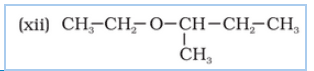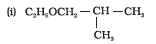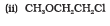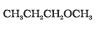Apply to Aakash iACST Scholarship Test 2024
NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers - This article covers NCERT Class 12 Chemistry solutions chapter 11. You are going to study the Chemistry of three classes of compounds which are alcohols, phenols and ethers and also this chapter will discuss the reactions involved in the preparation of alcohols, phenols and ethers.
Latest: JEE Main high scoring chapters
Don't Miss: JEE Main 10 year's papers
Recommended: NEET high scoring chapters
Suggested: NEET question papers with solutions
Alcohols are formed when a hydrogen atom in an aliphatic hydrocarbon is replaced by -OH group and Phenols are formed when a hydrogen atom in an aromatic hydrocarbon is replaced by –OH group while Ethers are formed by the substitution of an H-atom in a hydrocarbon by an alkoxy(R-O) or by an aryloxy(Ar-O) group. The NCERT solutions for Class 12 Chemistry chapter 11 Alcohols, Phenols and Ethers are prepared by Chemistry subject experts. These NCERT solutions help students in their preparation for the CBSE Board exam and in competitive exams like JEE Mains, NEET etc.
Also Read :
NCERT solutions for Class 12 Chemistry chapter 11 Alcohols, Phenols and Ethers start with the IUPAC nomenclature of alcohols, phenols and ethers followed by the topics:- reactions involved in the preparation of alcohols from aldehydes, ketones, alkenes and carboxylic acids and discuss reactions involved in the preparation of phenols from benzene sulphonic acids, haloarenes, cumene and diazonium salts. Alcohols Phenols and Ethers Class 12 also discuss reactions involved in the preparation of ethers from alcohols and alkyl halides. Scroll down to know more details about the NCERT solutions for Class 12 Chemistry Chapter 11 PDF download.
Find NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers Below:
Solutions to In-Text Questions Ex 11.1 to 11.12
Question 11. 1 (1) Classify the following as primary, secondary and tertiary alcohols:

Answer :
To classify we look at the OH bonded carbon.
Here, only 1 carbon is attached to it, hence it is primary alcohol .
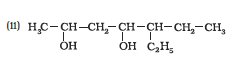
Question 11.1 (2) Classify the following as primary, secondary and tertiary alcohols:

Answer :
To classify we look at the OH bonded carbon.
Here, only 1 carbon is attached to it, hence it is primary alcohol.
Question 11.1 (3) Classify the following as primary, secondary and tertiary alcohols:
Answer :
To classify we look at the OH bonded carbon.
Here, only 1 carbon is attached to it, hence it is primary alcohol.
Question 11.1 (4) Classify the following as primary, secondary and tertiary alcohols:

Answer :
To classify we look at the OH bonded carbon.
Here, 2 carbons are attached to it, hence it is secondary alcohol.
Question 11.1 (5) Classify the following as primary, secondary and tertiary alcohols:

Answer :
To classify we look at the OH bonded carbon.
Here, 2 carbons are attached to it, hence it is secondary alcohol.
Question 11.1 (6) Classify the following as primary, secondary and tertiary alcohols :
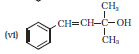
Answer:
To classify we look at the OH bonded carbon.
Here, 3 carbons are attached to it, hence it is tertiary alcohol.
Question 11.2 Identify allylic alcohols in the above examples.
Answer :
The alcohols (ii) and (vi) are allylic alcohols. Because -C=C-C-OH is the skeleton of allylic alcohol.
Question 11.3 (1) Name the following compounds according to IUPAC system.
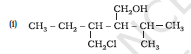
Answer :
3-Chloromethyl-2-isopropylpentan-1-ol
Question 11.3 (2) Name the following compounds according to IUPAC system.
Answer :
2, 5-Dimethylhexane-1, 3-diol
Question 11.3 (3) Name the following compounds according to IUPAC system.
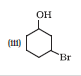
Answer :
3-Bromocyclohexanol
Question 11.3 (5) Name the following compounds according to IUPAC system.
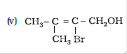
Answer :
2-Bromo-3-methylbut-2-en-1-ol
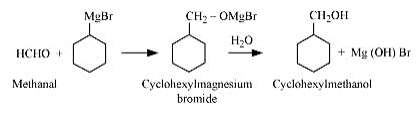
Question 11.4 (1) Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal ?
Answer :
The reaction of a suitable Grignard reagent on the methanal is mentioned below:
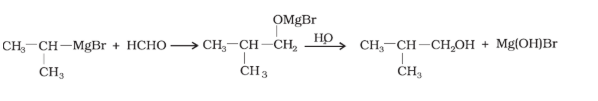
Question 11.4 (2) Show how are the following alcohols prepared by the reaction of a suitable
Grignard reagent on methanal ?
Answer :
Question 11.5 (2) Write structures of the products of the following reactions: 
Answer :
Product of the given reaction is-
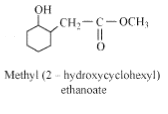
Question 11.5 (3) Write structures of the products of the following reactions:
Answer : 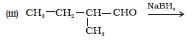
Product of the given reaction is
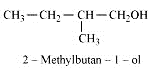
Question 11.6 (1) Give structures of the products you would expect when each of the following alcohol reacts with
Answer :
Primary alcohols do no react with Lucas’ reagent.
Hence no reaction.
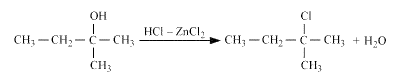
Question 11.6 (2) Give structures of the products you would expect when each of the following alcohol reacts with with 2-Methylbutan-2-ol
Answer :
Reaction of with 2-Methylbutan-2-ol
Question 11.6 (3) Give structures of the products you would expect when each of the following alcohol reacts with HBr with Butan-1-ol
Answer :
Reaction of HBr with Butan-1-ol
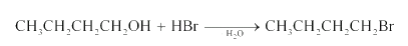
Question 11.6 (4) Give structures of the products you would expect when each of the following alcohol reacts with HBr with 2-Methylbutan-2-ol
Answer :
Reaction of HBr with 2-Methylbutan-2-ol
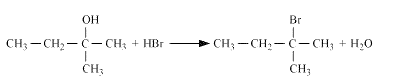
Question 11.6 (5) Give structures of the products you would expect when each of the following alcohol reacts with with Butan-1-ol
Answer :
Reaction of with Butan-1-ol
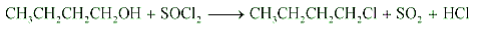
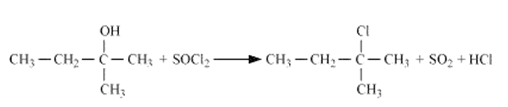
Question 11.6 (6) Give structures of the products you would expect when each of the following alcohol reacts with with 2-Methylbutan-2-ol
Answer :
Reaction of with 2-Methylbutan-2-ol
Question 11.7(1) Predict the major product of acid catalysed dehydration of 1-methylcyclohexanol
Answer :
Dehydration of 1-methylcyclohexanol
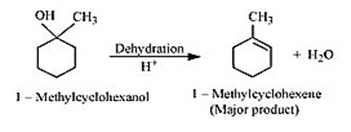
1-Methylcyclohexene is the major product.
Question 11.7 (2) Predict the major product of acid catalysed dehydration of butan-1-ol
Answer :
Dehydration of butan-1-ol
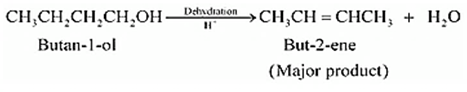
But-2-ene is the major product.
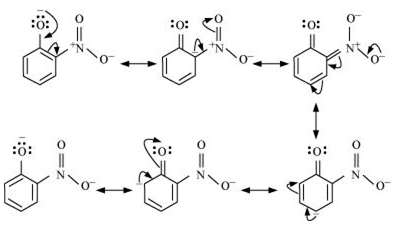
Question 11.8 Ortho and para nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide ions.
Answer :
Resonance structure of ortho-nitrophenol
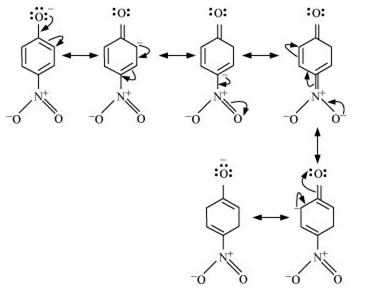
Resonance structure of para-nitrophenol
Question 11.9 (1) Write the equations involved in the following reactions: Reimer - Tiemann reaction
Answer :
Reimer - Tiemann reaction
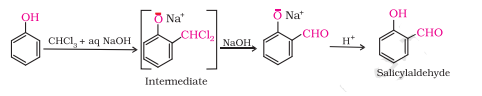
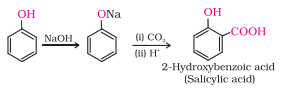
Question 11.9 (2) Write the equations involved in the following reactions: Kolbe’s reaction
Answer :
Kolbe’s reaction
Question 11.10 Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3-methylpentan-2-ol.
Answer :
1.Reaction of ethanol with hydrogen bromide.
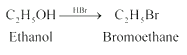
2. Reaction of 3-methylpentan-2-ol with sodium
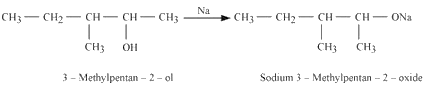
3. Reaction of product formed in 1st and reaction with the product formed in the 2nd reaction.
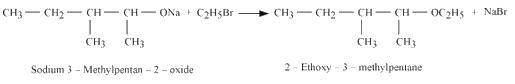
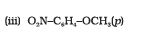
Question 11.11 Which of the following is an appropriate set of reactants for the preparation of 1-methoxy-4-nitrobenzene and why?

Answer :
(ii) is appropriate because is nucleophile whereas
is also a strong base. So elimination will be dominating in (i).
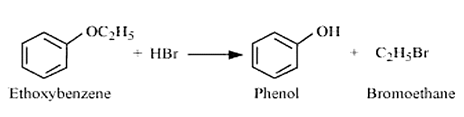
Question 11.12 (2) Predict the products of the following reactions:
Answer :
The reaction between ethoxybenzene and HBr is
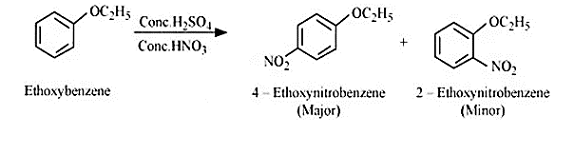
Question 11.12 (3) Predict the products of the following reactions:
Answer :
Reaction between ethoxybenzene and
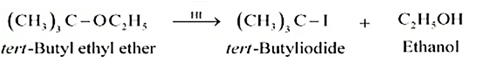
Question 11.12 (4) Predict the products of the following reactions:
Answer :
Reaction between ter - butyl ethyl ether and HI
NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers - Exercise Questions
Question 11.1 (1) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 2,2,4-Trimethylpentan-3-ol
Question 11.1 (2) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 5-Ethylheptane-2,4-diol
Question 11.1 (3) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is Butane-2,3-diol
Question 11.1 (4) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is Propane-1,2,3-triol
Question 11.1 (5) Write IUPAC names of the following compounds:
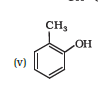
Answer :
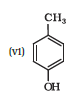
IUPAC name of the given compound is 2-Methylphenol
Question 11.1 (6) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 4-Methylphenol
Note : Also called p-cresol
Question 11.1 (7) Write IUPAC names of the following compounds:
Answer :
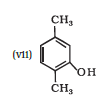
IUPAC name of the given compound is 2,5-Dimethylphenol
Question 11.1 (8) Write IUPAC names of the following compounds:
Answer :
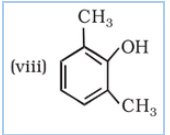
IUPAC name of the given compound is 2,6-Dimethylphenol
Question 11.1 (9) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 1-Methoxy-2-methylpropane
Question 11.1 (10) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is Ethoxybenzene
Question 11.1 (11) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 1-Phenoxyheptane
Question 11.1 (12) Write IUPAC names of the following compounds:
Answer :
IUPAC name of the given compound is 2-Ethoxybutane
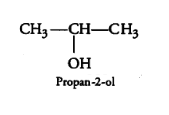
Question 11.2 Write structures of the compounds whose IUPAC names are as follows:
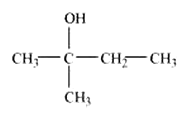
(i) 2-Methylbutan-2-ol
Answer :
Structure of 2-Methylbutan-2-ol
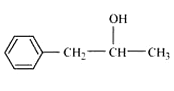
Question 11.2 Write structures of the compounds whose IUPAC names are as follows: (ii) 1-Phenylpropan-2-ol
Answer :
Structure of 1-Phenylpropan-2-ol
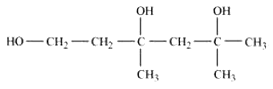
Question 11.2(iii) Write structures of the compounds whose IUPAC names are as follows: (iii) 3,5-Dimethylhexane –1, 3, 5-triol
Answer :
structure of 3,5-Dimethylhexane –1, 3, 5-triol
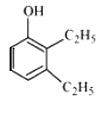
Question 11.2(iv) Write structures of the compounds whose IUPAC names are as follows: (iv) 2,3 – Diethylphenol
Answer :
structure of 2,3 – Diethylphenol
Question 11.2(v) Write structures of the compounds whose IUPAC names are as follows:
(v) 1 – Ethoxypropane
Answer :
structure of 1 – Ethoxypropane
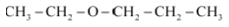
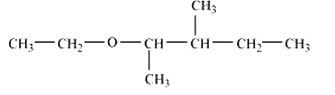
Question 11.2(vi) Write structures of the compounds whose IUPAC names are as follows:
(vi) 2-Ethoxy-3-methylpentane
Answer :
structure of 2-Ethoxy-3-methylpentane
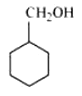
Question 11.2(vii) Write structures of the compounds whose IUPAC names are as follows:
(vii) Cyclohexylmethanol
Answer :
Structure of Cyclohexylmethanol
Question 11.2(viii) Write structures of the compounds whose IUPAC names are as follows:
(viii) 3-Cyclohexylpentan-3-ol
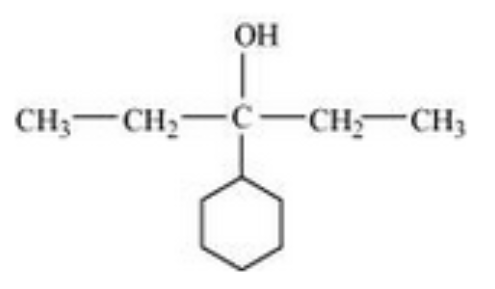
Answer :
The structure of 3-Cyclohexylpentan-3-ol is as follows:
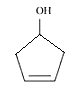
Question 11.2(ix) Write structures of the compounds whose IUPAC names are as follows:
(ix) Cyclopent-3-en-1-ol
Answer :
structure of Cyclopent-3-en-1-ol
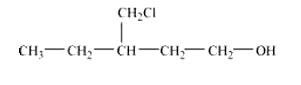
Question 11.2(x) Write structures of the compounds whose IUPAC names are as follows:
(x) 4-Chloro-3-ethylbutan-1-ol.
Answer :
structure of 4-Chloro-3-ethylbutan-1-ol
Question 11. 3 (i) Draw the structures of all isomeric alcohols of molecular formula
and give their IUPAC names.
Answer :
The structures of all isomeric alcohols of C 5 H 12 O are given below:
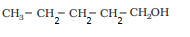 | Pentan-1-ol |
 | Pentan-2-ol |
 | Pentan-3-ol |
 | 3-Methylbutan-1-ol |
 | 3-Methylbutan-1-ol |
 | 2,2-Dimethylpropan-1-ol |
 | 3-Methylbutan-2-ol |
 | 2-Methylbutan-2-ol |
Question 11. 3 (ii) Classify the isomers of alcohols in question 11.3 (i) as primary, secondary and tertiary alcohols.
Answer :
Primary Alcohol: Pentan-1-ol; 2-Methylbutan-1-ol; 3-Methylbutan-1-ol; 2,2-Dimethylpropan-1-ol
Secondary Alcohol: Pentan-2-ol; 3-Methylbutan-2-ol; Pentan-3-ol
Tertiary Alcohol: 2-Methylbutan-2-ol
Question 11.4 Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Answer :
Propanol forms intermolecular H-bonds because of the presence of -OH group while butane cannot. To break these bonds, extra energy will be required. This causes a higher boiling point for propanol as compared to butane.
Question 11.5 Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Answer :
Alcohols form hydrogen bonds with water due to the presence of –OH group whereas hydrocarbons cannot. Due to this inter molecular hydrogen bonding, alcohols are more soluble in water.
Question 11.6 What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Answer :
Hydroboration-oxidation reaction also called HBO reaction is the addition of borane followed by oxidation to produce alcohol.
Eg: Hydroboration-oxidation reaction of propene. In this reaction, propene reacts with diborane (BH 3 ) 2 to form trialkyl borane. This addition product is oxidized by hydrogen peroxide in the presence of aqueous sodium hydroxide to form propan-1-ol.
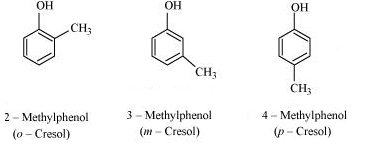
Question 11.7 Give the structures and IUPAC names of monohydric phenols of molecular formula, .
Answer :
The structures and IUPAC names of monohydric phenols of molecular formula, .
Phenol:
Answer :
Due to inter-molecular H bonding in para-nitrophenol, it gets tightly bonded with water. But ortho nitrophenol has intra-molecular H bonding and hence is steam volatile.
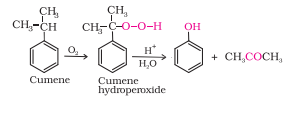
Question 11.9 Give the equations of reactions for the preparation of phenol from cumene.
Answer :
Cumene (isopropylbenzene) is oxidised in the presence of air to form cumene hydroperoxide. On treating with dilute acid it is converted to phenol and acetone.
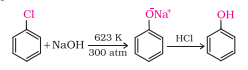
Question 11.10 Write chemical reaction for the preparation of phenol from chlorobenzene.
Answer :
Chlorobenzene, when fused with NaOH, produces sodium phenoxide which on acidification produces Phenol.
Question 11.11 Write the mechanism of hydration of ethene to yield ethanol.
Answer :
Ethanol is yielded from ethene by acid catalysed hydration.
The mechanism:
Step 1. Protonation of alkene to form carbocation by electrophilic attack of hydronium ion.
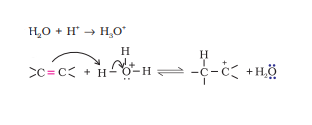
Step 2. Nucleophilic attack of water on carbocation .
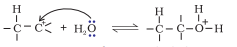
Step 3. Deprotonation to form an alcohol.
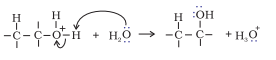
Question 11.12 You are given benzene, and NaOH. Write the equations for the preparation of phenol using these reagents .
Answer :
When benzene reacts with and heat it gives benzene sulphonic acid ,and after sulphonic this acid with NaOH then it gives phenol
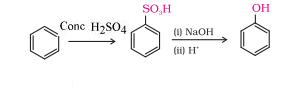
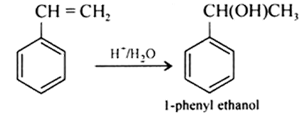
Question 11.13 Show how will you synthesise:
(i) 1-phenylethanol from a suitable alkene.
Answer :
Styrene on acid catalysed hydration gives 1-phenylethanol.
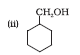
Question 11.13 (2) Show how will you synthesise:
(ii) cyclohexylmethanol using an alkyl halide by an SN2 reaction.
Answer :
On adding NaOH to chloromethylcyclohexane, cyclohexy methanol is formed.
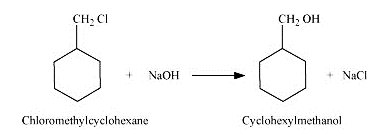
Question 11.13 Show how will you synthesise:
(iii) pentan-1-ol using a suitable alkyl halide?
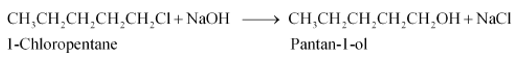
Answer :
when 1-chloropentane reacts with NaOH it gives pantan-1-ol
Question 11.14 Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
Answer :
1. Phenol reacts with sodium to give sodium phenoxide, liberating hydrogen gas.

2. Phenol reacts with sodium hydroxide to give sodium phenoxide and water.

Phenol is more acidic than ethanol. This is because phenol after losing a proton becomes phenoxide ion which is highly stable due to resonance whereas ethoxide ion does not.
Question 11.15 Explain why is ortho nitrophenol more acidic than ortho methoxyphenol ?
Answer :
Ortho-nitrophenol is more acidic than ortho- methoxyphenol . The presence of the nitro group, which is an electron withdrawing group, at the ortho position decreases the electron density in the O-H bond. Also, the o- nitrophenoxide ion formed after the loss of protons is stabile due to resonance. Hence, ortho nitrophenol is a stronger acid. Whereas the methoxy group is an electron-releasing group. Thus, it increases the electron density in the O-H bond.
Question 11.16 Explain how does the –OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Answer :
The -OH group is an electron-donating group (EDG). Thus, it increases the electron density in the benzene ring in the resonance structure of phenol. As a result, the benzene ring is activated towards electrophilic substitution.
Question 11.17(i) Give equations of the following reactions:
(i) Oxidation of propan-1-ol with alkaline solution.
Answer :
Oxidation of propane-1-ol with alkaline solution gives propanoic acid.
Question 11.17(ii) Give equations of the following reactions:
Answer :
Bromine in with phenol produces a mixture of o-bromo phenol and p-bromo phenol is formed.
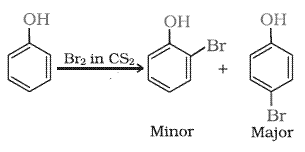
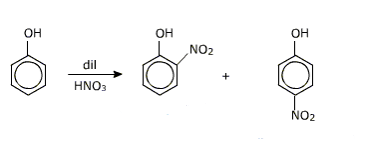
Question 11.17(iii) Give equations of the following reactions:
(iii) Dilute HNO3 with phenol.
Answer :
When dilute HNO3 reacts with phenol it gives o-bromo phenol and p-bromo phenol
Question 11.17(iv) Give equations of the following reactions:
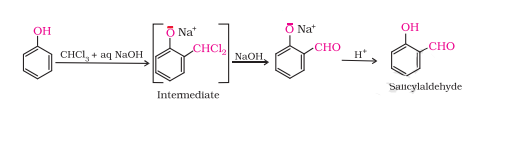
(iv) Treating phenol with chloroform in presence of aqueous NaOH.
Answer :
Treating phenol with chloroform in presence of aqueous NaOH.
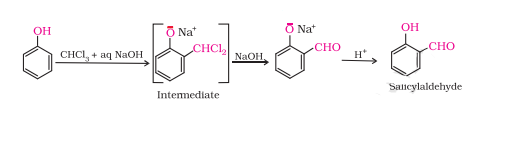
This reaction is known as the Reimer-Tiemann reaction.
Question 11.18(i) Explain the following with an example.
(i) Kolbe’s reaction.
Answer :
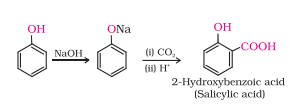
Kolbe’s reaction: Phenol with carbon dioxide under pressure followed by treating the product with sulphuric acid produces Ortho-hydroxybenzoic acid (salicylic acid). Phenoxide ion generated is more reactive than phenol towards electrophilic aromatic substitution. Hence, it undergoes electrophilic substitution with carbon dioxide, a weak electrophile.
Question 11.18(ii) Explain the following with an example.
(ii) Reimer-Tiemann reaction.
Answer :
On treating phenol with chloroform in the presence of sodium hydroxide, a –CHO group is introduced at the ortho position of the benzene ring. This reaction is known as Reimer - Tiemann reaction
Question 11.18(iii) Explain the following with an example. (iii) Williamson ether synthesis.
Answer :
Williamson ether synthesis is a reaction forming ether from a primary alkyl halide via S N 2 reaction.
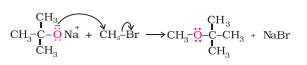
Question 11.18(iv) Explain the following with an example.(iv) Unsymmetrical ether.
Answer :
If the alkyl or aryl groups attached to the oxygen atom are different, then it is mixed or unsymmetrical ether.
Eg:
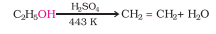
Question 11.19 . Write the mechanism of acid dehydration of ethanol to yield ethene.
Answer :
Alcohols undergo dehydration (removal of a molecule of water) to form alkenes on treating with a protic acid. Ethanol undergoes dehydration by heating it with concentrated sulphuric acid at 443 K.
Question 11.20(i) How are the following conversions carried out?
(i) Propene Propan-2-ol.
Answer :
Acid catalysed hydration of propene produces propan-2-ol.
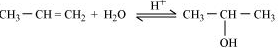
Question 11.20(ii) How are the following conversions carried out?
(ii) Benzyl chloride Benzyl alcohol.
Answer :
Benzyl chloride treated with NaOH followed by acidification produces benzyl alcohol.

Question 11.20(iii) How are the following conversions carried out?
(iii) Ethyl magnesium chloride Propan-1-ol.
Answer :
Ethyl magnesium chloride treated with formaldehyde followed by hydrolysis produces propan-1-ol.
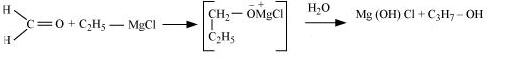
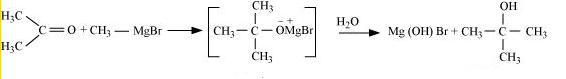
Question 11.20(iv) How are the following conversions carried out?
(iv) Methyl magnesium bromide 2-Methylpropan-2-ol.
Answer :
Methyl magnesium bromide treated with propane, gives 2-methylpropane-2-ol on hydrolysis.
Question 11.21(i) Name the reagents used in the following reactions:
(i) Oxidation of a primary alcohol to carboxylic acid.
Answer :
Acidic/neutral/alkaline potassium permanganate or acidified
Question 11.21(ii) Name the reagents used in the following reactions:
(ii) Oxidation of a primary alcohol to aldehyde.
Answer :
The reagent used for oxidation of primary alcohol to aldehyde is Pyridinium chlorochromate (PCC) .
Question 11.21(iii) Name the reagents used in the following reactions:
(iii) Bromination of phenol to 2,4,6-tribromophenol.
Answer :
Reagents used in the bromination of phenol to 2,4,6-tribromophenol is Bromine water
Question 11.21(iv) Name the reagents used in the following reactions:
(iv) Benzyl alcohol to benzoic acid.
Answer :
Reagent used in the benzyl alcohol to benzoic acid isAcidified (potassium permanganate)
Question 11.21(v) Name the reagents used in the following reactions:
(v) Dehydration of propan-2-ol to propene.
Answer :
Reagents used in the dehydration of propan-2-ol to propene is Concentrated Phosphoric acid.
Question 11.21(vi) Name the reagents used in the following reactions:
(vi) Butan-2-one to butan-2-ol.
Answer :
Reagent used in the butan-2-one to butan-2-ol is
Question 11.22 Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Answer :
Ethanol undergoes intermolecular hydrogen bonding due to the presence of -OH group. Therefore, extra energy is required to break those hydrogen bonds. Whereas methoxymethane does not make H-bonds and hence ethanol has a higher boiling point than methoxymethane.
Question 11.23(i) Give IUPAC names of the following ethers:
Answer :
IUPAC names of the given ether is 1-Ethoxy-2-methylpropane
Question 11.23(ii) Give IUPAC names of the following ethers:
Answer :
IUPAC names of the given ether is 2-Chloro-1-methoxyethane
Question 11.23(iii) Give IUPAC names of the following ethers:
Answer :
IUPAC names of the given ether is 4-Nitroanisole
Question 11.23(iv) Give IUPAC names of the following ethers:
Answer :
IUPAC names of the given ether is 1-Methoxypropane
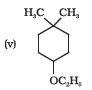
Question 11.23(v) Give IUPAC names of the following ethers:
Answer :
IUPAC names of the given ether is 4-Ethoxy-1, 1-dimethylcyclohexane
Question 11.23(vi) Give IUPAC names of the following ethers:
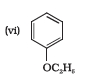
Answer :
IUPAC names of the given ether is Ethoxybenzene
Question 11.24(i) Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis:
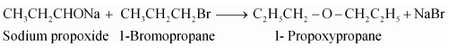
(i) 1-Propoxypropane
Answer :
Names of reagents and equations for the preparation of the 1-Propoxypropane ether by Williamson’s synthesis:-
Question 11.24(ii) Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis:
(ii) Ethoxybenzene
Answer :
Names of reagents and equations for the preparation of the Ethoxybenzene ether by Williamson’s synthesis:-
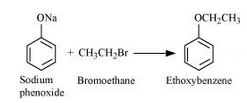 with NaBr as side product.
with NaBr as side product.
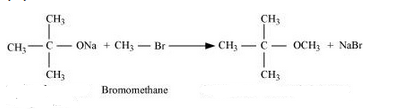
Question 11.24(iii) Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis:
(iii) 2-Methoxy-2-methylpropane
Answer :
Names of reagents and equations for the preparation of the2-Methoxy-2-methylpropane ether by Williamson’s synthesis:-
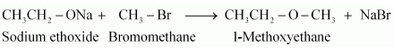
Question 11.24(iv) Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis: (iv) 1-Methoxyethane
Answer :
Names of reagents and equations for the preparation of the1-Methoxyethane ether by Williamson’s synthesis:-
Question 11.25 Illustrate with examples the limitations of Williamson synthesis for the preparation of certain types of ethers.
Answer :
Williamson synthesis involves attack by alkoxide ion on a primary alkyl halide. But if secondary or tertiary alkyl halides are taken then alkenes would be produced because elimination would take place. This is because alkoxides are nucleophiles as well as strong bases.

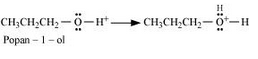
Question 11.26 How is 1-propoxypropane synthesised from propan-1-ol? Write mechanism of this reaction.
Answer :
Propan-1-ol on dehydration using protic acids such as sulphuric acid gives 1-propoxypropane.
Mechanism of this reaction:
Formation of protonated alcohol.
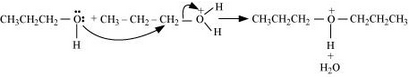
Formation of carbocation: It is the slowest step and hence, the rate determining step of the reaction.
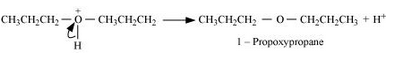
Formation of ethene by the elimination of a proton.
Question 11.27 Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Answer :
The formation of ethers by dehydration of a primary alcohol is an S N 2 reaction. In case of secondary or tertiary alcohols, the alkyl group is hindered and hence elimination dominates substitution. Therefore alkenes are formed in place of ethers.
Question 11.28(i) Write the equation of the reaction of hydrogen iodide with:
(i) 1-propoxypropane
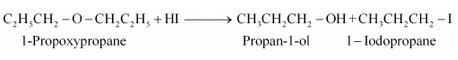
Answer :
1-propoxypropane reacts with HI to give propan-1-ol and 1-iodopropane as the products.
Question 11.28(ii) Write the equation of the reaction of hydrogen iodide with: (ii) methoxybenzene
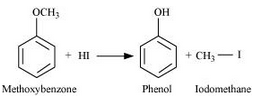
Answer :
Methoxybenzene reacts with HI to give phenol and iodomethane.
Question 11.28(iii) Write the equation of the reaction of hydrogen iodide with: (iii) benzyl ethyl ether.
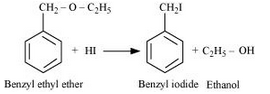
Answer :
Benzyl ethyl ether reacts with HI to give benzyl iodide and ethanol.
Question 11.29(i) Explain the fact that in aryl alkyl ethers (i) the alkoxy group activates the benzene ring towards electrophilic substitution
Answer :
Due to the +R effect of the alkoxy group, it increases the electron density of the benzene ring pushing electrons into the ring making the benzene ring activated towards electrophilic substitution reactions.
Question 11.29(ii) Explain the fact that in aryl alkyl ethers (ii) it directs the incoming substituents to ortho and para positions in benzene ring.
Answer :
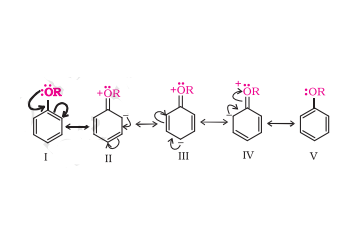
The above resonating structures shows that the electron density increases more at the ortho and para positions as compared to the meta positions. Hence, the alkoxy group directs the incoming substituents to ortho and para positions in the benzene ring.
Question 11.30. Write the mechanism of the reaction of HI with methoxymethane.
Answer :
Following is the mechanism:
1. Protonation of methoxymethane
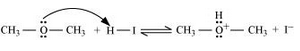
2. Nucleophilic attack of
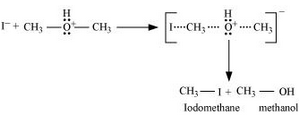
3. If HI is in excess, then methanol formed in step 2 reacts with another HI molecule and gets converted to methyl iodide at a high temperature.
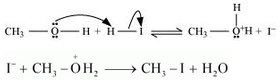
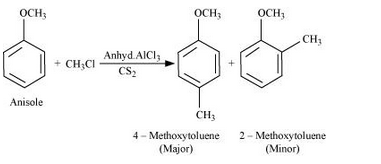
Question 11.31(i) Write equations of the following reactions:
(i) Friedel-Crafts reaction – alkylation of anisole.
Answer :
Fridel Craft reaction(Alkylation):
Question 11.31(ii) Write equations of the following reactions: (ii) Nitration of anisole.
Answer :
Nitration of anisole:
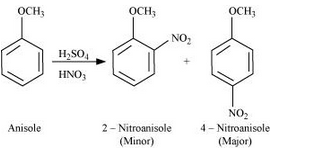
Question 11.31(iii) Write equations of the following reactions: (iii) Bromination of anisole in ethanoic acid medium.
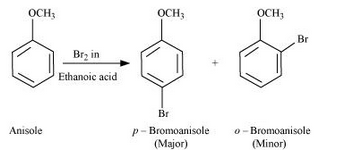
Answer :
Bromination of anisole in ethanoic acid medium:
Question 11.31(iv) Write equations of the following reactions: (iv) Friedel-Craft’s acetylation of anisole.
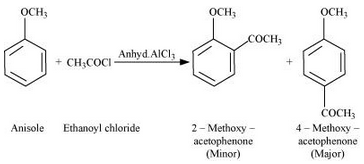
Answer :
Friedel-Craft’s acetylation of anisole:
Question 11.32 Show how would you synthesise the following alcohols from appropriate alkenes?
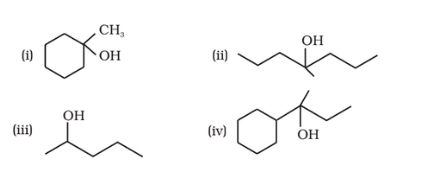
![]()
Answer :
(i) 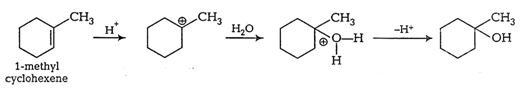
(ii) 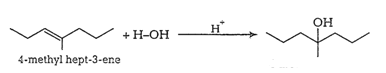
(iii) 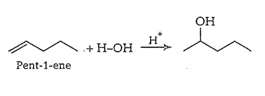
(iv) 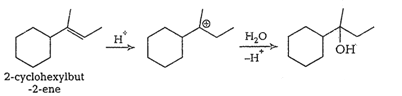
Question 11.33 When 3-methylbutan-2-ol is treated with HBr, the following reaction takes
place:
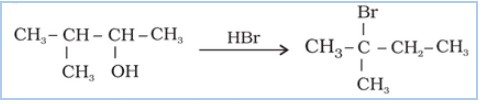
![]()
Give a mechanism for this reaction.
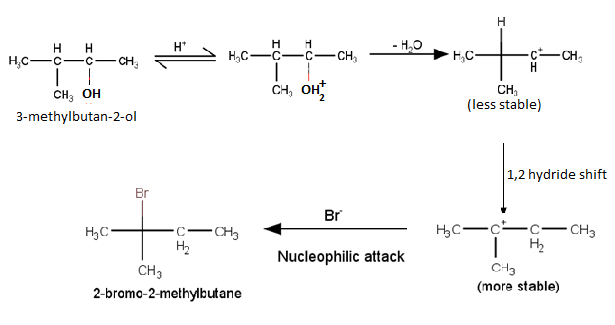
(Hint : The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.
Answer :
Mechanism for the reaction 3-methylbutan-2-ol is treated with HBr
Chemistry Chapter 11 NCERT Solutions Insights
Alcohols Phenols and Ethers Class 12 NCERT solutions has answers to a total of 12 topic wise questions and 33 questions in the exercise. In the CBSE boards exam, the weightage of this chapter is 4 marks hence it is recommended to solve all the exercises of the book to get good marks. You will find all the NCERT solutions for Class 12 Chemistry chapter 11 Alcohols, Phenols and Ethers here free of cost.
By referring to the NCERT solutions for class 12, students can understand all the important concepts and practice questions well enough before their examination. Alcohols are the basic compound for the formation of detergents, phenols are the basic compound for the formation of antiseptics and ethers is the basic compound for the formation of fragrances.
Topics and Sub-topics of NCERT Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers-
11.1 Classification
11.2 Nomenclature
11.3 Structures of Functional Groups
11.4 Alcohols and Phenols
11.5 Some Commercially Important Alcohols
11.6 Ethers
NCERT Solutions Class 12 Chemistry Chapter Wise
| Chapter 1 | The Solid State |
| Chapter 2 | Solutions |
| Chapter 3 | Electrochemistry |
| Chapter 4 | Chemical Kinetics |
| Chapter 5 | Surface chemistry |
| Chapter 6 | General Principles and Processes of isolation of elements |
| Chapter 7 | The P-block elements |
| Chapter 8 | The d and f block elements |
| Chapter 9 | Coordination compounds |
| Chapter 10 | Haloalkanes and Haloarenes |
| Chapter 11 | Alcohols, Phenols and Ethers |
| Chapter 12 | Aldehydes, Ketones and Carboxylic Acids |
| Chapter 13 | Amines |
| Chapter 14 | Biomolecules |
| Chapter 15 | Polymers |
| Chapter 16 | Chemistry in Everyday life |
NCERT Solutions for Class 12 Subject wise
- NCERT Solutions for class 12 biology
- NCERT solutions for class 12 maths
- NCERT solutions for class 12 chemistry
- NCERT Solutions for class 12 physics
SAT® | CollegeBoard
Registeration closing on 19th Apr for SAT® | One Test-Many Universities | 90% discount on registrations fee | Free Practice | Multiple Attempts | no penalty for guessing
TOEFL ® Registrations 2024
Thinking of Studying Abroad? Think the TOEFL® test. Save 10% on your TOEFL exam with ApplyShop gift cards!
Benefits of NCERT Solutions for class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
- The answers presented in a comprehensive manner in the NCERT solutions for class 12 chemistry chapter 11 Alcohols, Phenols and Ethers will help in understanding chapter easily.
- It will be easy to revise because the detailed solutions will help you to remember the concepts and fetch you good marks.
- Homework problems won't bother you anymore, all you need to do is check the detailed NCERT solutions for class 12 chemistry chapter 11 Alcohols, Phenols and Ethers and you are good to go.
If you have a doubt or question that is not available here or in any of the chapters, contact us. You will get all the answers that will help you score well in your exams.
Also Check NCERT Books and NCERT Syllabus here:
Frequently Asked Question (FAQs)
For complete solutions refer to this website: https://school.careers360.com/ncert/ncert-solutions-class-12-chemistry
Important topics:
- Chemical Reactions of Alcohols, Phenols & Ethers
- Physical Properties of Alcohols, Phenols and Ethers
- Preparation of Alcohols
- Chemical Reactions of Ethers
- Physical Properties of Ethers
- Preparation of Ethers
- Preparation of Phenols
- Some Commercially Important Alcohols
- Introduction and Classification of Alcohols, Phenols & Ethers
- Nomenclature
This chapter holds weightage of 4 percent in NEET examination. Practice NEET previous year papers, NCERT question and NCERT exemplar questions for a good score.
Questions worth 5-6 marks are asked from this chapter in CBSE Board exam. Follow the NCERT syllabus for good score in board exam.
This chapter holds the weightage of 4-5 Marks in JEE Main
Also Read
Articles
Certifications By Top Providers
Explore Top Universities Across Globe
Questions related to CBSE Class 12th
Hello aspirant,
The purpose of graphic design extends beyond the brand's look. Nevertheless, by conveying what the brand stands for, it significantly aids in the development of a sense of understanding between a company and its audience. The future in the field of graphic designing is very promising.
There are various courses available for graphic designing. To know more information about these courses and much more details, you can visit our website by clicking on the link given below.
https://www.careers360.com/courses/graphic-designing-course
Thank you
Hope this information helps you.
hello,
Yes you can appear for the compartment paper again since CBSE gives three chances to a candidate to clear his/her exams so you still have two more attempts. However, you can appear for your improvement paper for all subjects but you cannot appear for the ones in which you have failed.
I hope this was helpful!
Good Luck
Hello dear,
If you was not able to clear 1st compartment and now you giving second compartment so YES, you can go for your improvement exam next year but if a student receives an improvement, they are given the opportunity to retake the boards as a private candidate the following year, but there are some requirements. First, the student must pass all of their subjects; if they received a compartment in any subject, they must then pass the compartment exam before being eligible for the improvement.
As you can registered yourself as private candidate for giving your improvement exam of 12 standard CBSE(Central Board of Secondary Education).For that you have to wait for a whole year which is bit difficult for you.
Positive side of waiting for whole year is you have a whole year to preparing yourself for your examination. You have no distraction or something which may causes your failure in the exams. In whole year you have to stay focused on your 12 standard examination for doing well in it. By this you get a highest marks as a comparison of others.
Believe in Yourself! You can make anything happen
All the very best.
Hello Student,
I appreciate your Interest in education. See the improvement is not restricted to one subject or multiple subjects and we cannot say if improvement in one subject in one year leads to improvement in more subjects in coming year.
You just need to have a revision of all subjects what you have completed in the school. have a revision and practice of subjects and concepts helps you better.
All the best.
Hi,
You just need to give the exams for the concerned two subjects in which you have got RT. There is no need to give exam for all of your subjects, you can just fill the form for the two subjects only.
Popular CBSE Class 12th Questions
Colleges After 12th
Also Read
Applications for Admissions are open.

JEE Main Important Physics formulas
ApplyAs per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

UPES School of Liberal Studies
ApplyRanked #52 Among Universities in India by NIRF | Up to 30% Merit-based Scholarships | Lifetime placement assistance

Aakash iACST Scholarship Test 2024
ApplyGet up to 90% scholarship on NEET, JEE & Foundation courses

JEE Main Important Chemistry formulas
ApplyAs per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

PACE IIT & Medical, Financial District, Hyd
ApplyEnrol in PACE IIT & Medical, Financial District, Hyd for JEE/NEET preparation

ALLEN JEE Exam Prep
ApplyStart your JEE preparation with ALLEN
Applications for CBSE Class 12 supplementary exams 2023 have started. Read details here.
CBSE Class 12th result 2023 declared. Check now.