Apply to Aakash iACST Scholarship Test 2024
NCERT Solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
NCERT solutions for Class 12 Chemistry chapter 12 Aldehydes, Ketones and Carboxylic Acids is an important chapter as it carries 6 out of 70 marks in the CBSE board examination. It is advisable to go through the Class 12 Chemistry chapter 12 Aldehydes Ketones and Carboxylic Acid to score well in the exams. The NCERT solutions provided here are completely free. By referring to the NCERT solutions for class 12, students can understand the concepts and practice questions for the examination. Class 12 chemistry chapter 12 NCERT solutions boosts confidence of students.
Latest: JEE Main high scoring chapters
Don't Miss: JEE Main 10 year's papers
Recommended: NEET high scoring chapters
Suggested: NEET question papers with solutions

Also Read,
- NCERT Exemplar Solutions For Aldehydes Ketones and Carboxylic Acid
- Aldehydes Ketones and Carboxylic Acid NCERT Chapter Notes
Find NCERT solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Solutions to In-Text Questions Ex 12.1 to 12.8
Question 12.1(i) Write the structures of the following compounds.
Answer :
The structure of the compound -methoxy propionaldehyde is shown here-

Question 12.1(ii) Write the structures of the following compounds.
Answer :
The structure of the compound 3-Hydroxy butanal is shown here-

Question 12.1(iii) Write the structures of the following compounds.
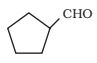
2-Hydroxy cyclopentane carbaldehyde
Answer :
The structure of the compound 2-Hydroxycyclopentane carbaldehyde is shown here-

Question 12.1(iv) Write the structures of the following compounds.
Answer :
The structure of the compound 4-oxopentanal is shown here-

Question 12.1(v) Write the structures of the following compounds.
Answer :
The structure of the compound Di-sec. butyl ketone is shown here-
![]()
Question 12.1(vi) Write the structures of the following compounds.
Answer :
The structure of the compound 4-Fluoro acetophenone is shown here-

Question 12.2(i) Write the structures of products of the following reactions:
(i) 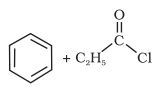
Answer :
When benzene is treated with acid chloride in the presence of anhydrous aluminum chloride group attached to the benzene ring. this reaction is known as friedel craft acylation reaction.

Question 12.2(ii) Write the structure of products of the following reactions;
(ii)
Answer :
Reaction of acyl chloride with dialkylcadmium ( ), prepared from reaction of cadmium chloride and grignard reagents ,gives ketone

Question 12.2(iii) Write the structures of products of the following reactions:
(iii)
Answer :
When propyne reacts with in presence of dil. sulphuric acid, water molecules as a nucleophile attack on suitable postion[
, primary anion are stable] and then tautomerisation occurs to get the final product

Question 12.2(iv) Write the structures of products of the following reactions:
(iv) 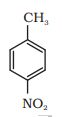
Answer :
Chromyl chloride oxidise the methyl group into a chromium complex, which on hydrolysis give aldehyde group.

Question 12.3 Arrange the following compounds in increasing order of their boiling points.
Answer :
Increasing order in their boiling points-
Alcohol has the highest boiling point due to more extensive intermolecular H-bonding. Aldehyde is more polar than ether so, ethanal has high BP than ethyl ether. And alkane has the lowest BP.
Question 12.4(i) Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
Ethanal, Propanal, Propanone, Butanone.
Answer :

By the above structure, we can see that, due to +I effect of alkyl group te electron density at Carbonyl carbon increase from ethanal to bytanone. And so the tendency of attacking nucleophile is decreased.
Thus the increasig order (reactivity towards nucleophile)-
Butanone < propanone < propanal < ethanal
Question 12.4(ii) Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
Benzaldehyde, p-Tolualdehyde, p-nitrobenzaldehyde, Acetophenone.
Answer :
If +I effect is more its reactivity towards nucleophilic addition is less. and -I is more, more reactive toward addition.

So, according to this concept, increasing order of their reactivity in nucleophilic addition reactions-
Acetophenone < p-tolualdehyde < benzaldehyde <p-nitrobenzaldehyde
Question 12.5(i) Predict the products of the following reactions:
(i) 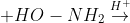
Answer :
The product of the reaction is-
Water molecule is removed as a by-product in this reaction.

Question 12.5(ii) Predict the products of the following reactions:
(ii) 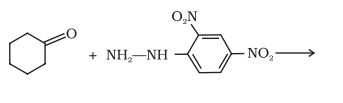 →
→
Answer :
The product of the reaction is a hydrazone, which is formed when ketone and 2, 4-dinitrophenyl hydrazine derivative reacts with each other.

Question 12.5(iii) Predict the products of the following reactions:
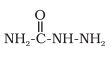
Answer :
The product of the above reaction is -
Water molecule is eleminated as a by-product in this reaction

Question 12.5(iv) Predict the products of the following reactions:
(iv) 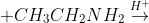
Answer :
The product of the above reaction is -
water is also obtained in this reaction as a by-product

Question 12.6(i) Give the IUPAC names of the following compounds:
Answer :
The IUPAC name of the compound is-
3-phenyl propanoic acid
Question 12.6(ii) Give the IUPAC names of the following compounds:
Answer :
The IUPAC name of the compound is -
3-methyl but-2-en-1-oic acid
Question 12.6(iii) Give the IUPAC names of the following compounds:
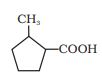
Answer :
The IUPAC name of the compound is-
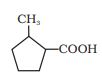 2-methyl cyclopentane carboxylic acid
2-methyl cyclopentane carboxylic acid
Question 12.6(Iv) Give the IUPAC names of the following compounds:
(iv) 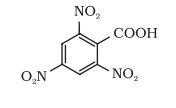
Answer :
The IUPAC name of the compound is-
2, 4, 6-trinitrobenzoic acid
Question 12.7(i) Show how each of the following compounds can be converted to benzoic acid?
Answer :
Strong oxidising agents like potassium permanganate in the presence of KOH, followed by acidic hydrolysis give benzoic acid.

Question 12.7(ii) Show how each of the following compounds can be converted to benzoic acid.
Answer :
On strong oxidation with potassium permanganate in presenc of KOH followed by acidic hydrolysis, give benzoic acid 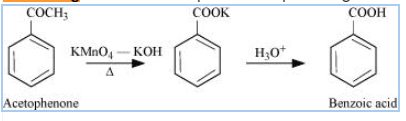
![]()
Question 12.7(iii) Show how each of the following compounds can be converted to benzoic acid?
Answer :
Make bromobenzene first Grignard reagent, react it with dry ice (carbon dioxide) followed by acidic hydrolysis gives benzoic acid.

Question 12.7(iv) Show how each of the following compounds can be converted to benzoic acid?
Answer :
On strong oxidation with potassium permanganate ( ) in the presence of strong alkali (KOH) followed by acidic hydrolysis gives benzoic acid.

Question 12.8(i) Which acid of each pair shown here would you expect to be stronger?
or
Answer :
is stronger than
due to -I effect of Fluorine decreases the electron density at OH bond, which makes it easier to lose proton (
). The conjugate base of
is more stable than
.
Question 12.8(ii) Which acid of each pair shown here would you expect to be stronger?
or
Answer :
is a stronger acid.
Fluorine has more -I effect than chlorine. So, can release proton easily than
.
Question 12.8(iii) Which acid of each pair shown here would you expect to be stronger?
(iii) or
Answer :
Since we know that inductive effect depends on distance. Greater is the distance lesser is the effect. So, -I effect in is more.
is more acidic than
.
Question 12.8(iv) Which acid of each pair shown here would you expect to be stronger?
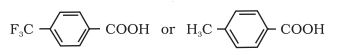
Answer :
Due to -I effect of Fluorine in A, it is easy to release proton ( ) but in compound B due to +I effect of methyl group it becomes difficlt te release protons. Therefore, compound A is more acidic than compound B.

Question: 12.1 (i). What is meant by the following terms ? Give an example of the reaction in each case.
Answer:
Cyanohydrin -
When ketone and aldehyde react with the hydrogen cyanide ( ) to yield cyanohydrin. The Reaction is very slow with the pure HCN, so it can be catalyzed by using a base.

Q 12.1 (ii). What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Acetal -
When aldehyde reacts with one molecule of monohydric alcohol in presence of dry HCl, it gives intermediate compound known as hemiacetals, which on further reaction with one more molecule of alcohol gives a product (gem-dialkoxy compound), known as acetal.
For example:

Q 12.1(iii). What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Semicarbazone -
This is the derivative of the aldehyde and ketone and it is derived from the condensation reaction between aldehyde and ketone. For example:

Q 12.1(iv). What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Aldol -
-hydroxy aldehyde is known as aldol and it can be prepared from condensation of aldehyde and ketone, having atleast one alpha-hydrogen atom in presence of dil. alkali as a catalyst.
For example:

Q 12.1(v) What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Hemiacetal -
Aldehyde reacts with one equivalent of a monohydric alcohol, to yield an alkoxy alcohol intermediate compound, in presence of dry hydrochloric acid. The intermediate is called hemiacetal.
For example:

Q 12.1 (vi) What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Oxime -
Aldehyde and ketone on reacting with the hydroxylamine in a weakly basic medium give oximes.
The general form of oxime-

For example:

Q 12.1 (vii) What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Ketal-
It is a cyclic compound, which is formed when a ketone reacts with the ethylene glycol in the presence of dry hydrochloric acid.
For example:

Q 12.1(vii) What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Imine -
These are the chemical compounds having carbon-nitrogen double bonds. It is formed when aldehyde and ketone react with ammonia and its derivatives.
For example:

Q 12.1(ix) What is meant by the following terms? Give an example of the reaction in each case.
Answer:
2,4-DNP-derivative-
These are produced when aldehyde and ketone are reacted with the 2, 4-dinitrophenylhydrazine in a weak basic medium. 2, 4DNP test is used for distinguishing between aldehyde and ketone.
For example:

Q 12.1 (x) What is meant by the following terms? Give an example of the reaction in each case.
Answer:
Schiff’s base-
When aldehyde and ketone are treated with the primary aliphatic or aromatic amines in the presence of acid produces Schiff's base.
For example:
![]()
Q 12.2(i) Name the following compounds according to IUPAC system of nomenclature:
Answer:
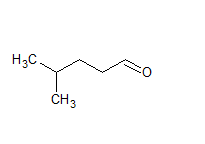 IUPAC name of this compound is 4-methyl pentanal
IUPAC name of this compound is 4-methyl pentanal
Q 12.2(ii) Name the following compounds according to IUPAC system of nomenclature:
Answer:
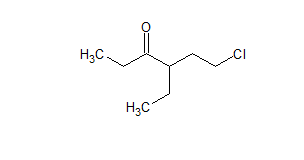 The IUPAC name of the compound is 6-chloro-4ethyl hexane-3-one
The IUPAC name of the compound is 6-chloro-4ethyl hexane-3-one
Q 12.2(iii) Name the following compounds according to IUPAC system of nomenclature:
Answer:
the structure of the compound is
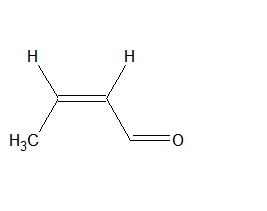 The IUPAC name of the compound is But -2-en-1-al
The IUPAC name of the compound is But -2-en-1-al
Q 12.2(iv) Name the following compounds according to IUPAC system of nomenclature:
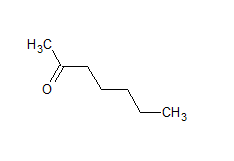
Answer:
The structure of the given compound is-
 The IUPAC name of the compound is - pentan-2, 4-dione.
The IUPAC name of the compound is - pentan-2, 4-dione.
Q 12.2 (v) Name the following compounds according to IUPAC system of nomenclature:
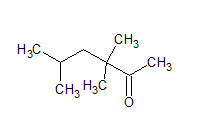
Answer:
The structure of the compound is -
 The IUPAC name of the given compound is 3, 3, 5-trimethyl hexan-2-one .
The IUPAC name of the given compound is 3, 3, 5-trimethyl hexan-2-one .
Q 12.2 (vi) Name the following compounds according to IUPAC system of nomenclature:
Answer:
The structure of the compound is -
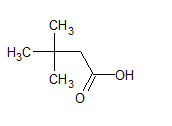 The IUPAC name of the compound is 3, 3-dimethyl butanoic acid .
The IUPAC name of the compound is 3, 3-dimethyl butanoic acid .
Q 12.2 (vii) Name the following compounds according to IUPAC system of nomenclature:
Answer:
The structure of the compound is-
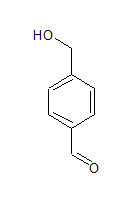 The IUPAC name of the compound is Benzene-1, 4-dicarbaldehyde .
The IUPAC name of the compound is Benzene-1, 4-dicarbaldehyde .
Q 12.3(i) Draw the structures of the following compounds.
Answer:
The structure of the 3-methylbutanal is shown here-

Q 12.3 (ii) Draw the structures of the following compounds.
Answer:
The structure of p-nitro propiophenone is shown here-

Q 12.3 (iii) Draw the structures of the following compounds.
Answer:
The strcuture of the -methyl benzaldehyde is shown here-

Q 12.3(iv) Draw the structures of the following compounds.
Answer:
The structure of the compound 4-methylpent-3-en-2-one is shown here-
![]()
Q 12.3(v) Draw the structures of the following compounds.
Answer:
The structure of the compound 4-chloro-pentan-2-one is shown here-

Q 12.3 (vi) Draw the structures of the following compounds.
3-Bromo-4-phenylpentanoic acid
Answer:
The structure of the compound 3-Bromo-4-phenyl pentanoic acid is shown here-
![]()
Q 12.3 (vii) Draw the structures of the following compounds.
Answer:
The structure of the compound p,p’-dihydroxy benzophenone acid is shown here-

Q 12.3 (viii) Draw the structures of the following compounds.
Answer:
The structure of the compound Hex-2-en-4-ynoic acid acid is shown here-

Answer:
The structure of the compound is
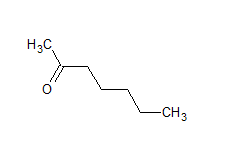 The IUPAC name of the compound is hept-2-one Common name is methyl-n-propyl ketone
The IUPAC name of the compound is hept-2-one Common name is methyl-n-propyl ketone
Q 12.4 (ii) Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
Answer:
The structure of the compound is
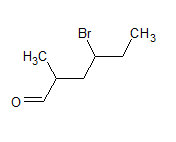 The IUPAC name of the compound is 4-bromo-2-methylhexanal
The IUPAC name of the compound is 4-bromo-2-methylhexanal
The common name is - -bromo-
-methyl-caproaldehyde
Q 12.4 (iii) Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
Answer:
structure os the compound is
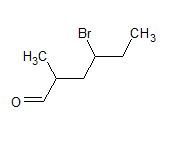 The IUPAC name of the compound is heptanal
The IUPAC name of the compound is heptanal
Q 12.4 (iv) Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
Answer:
structur eof the compound is
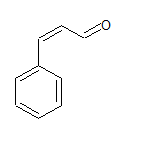 The IUPAC name of the sompound is 3-phenylprop-2-ene-al
The IUPAC name of the sompound is 3-phenylprop-2-ene-al
Common name is -phenylacrolein
Q 12.4 (v). Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
Answer:
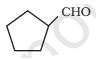 The IUPAC name of the compound is cyclopentane carbaldehyde.
The IUPAC name of the compound is cyclopentane carbaldehyde.
Q 12.4 (vi) Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
Answer:
structure of the compound is
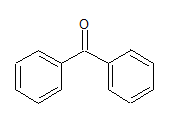 The IUPAC name of the compound is Diphenyl mentanone.
The IUPAC name of the compound is Diphenyl mentanone.
The common name is - benzophenone
Q 12.5 (i) Draw structures of the following derivatives.
The 2,4-dinitro phenyl hydrazone of benzaldehyde
Answer:
The structure of 2,4-dinitro phenyl hydrazone of benzaldehyde

12.5 (ii) Draw structures of the following derivatives.
Answer:
The structure of the Cyclopropanone oxime

Q 12.5 (iii) Draw structures of the following derivatives.
Answer:
The structure of Acetaldehydedimethylacetal

Q 12.5 (iv) Draw structures of the following derivatives.
The semicarbazone of cyclobutanone
Answer:
The structure of the semicarbazone of cyclobutanone

Q 12.5 (v) Draw structures of the following derivatives.
The ethylene ketal of hexan-3-one
Answer:
The structure of the ethylene ketal of hexan-3-one

Q 12.5 (vi) Draw structures of the following derivatives.
The methyl hemiacetal of formaldehyde
Answer:
The structure of the compound methyl hemiacetal of formaldehyde

Q 12.6 (i) Predict the products formed when cyclohexane carbaldehyde reacts with following reagents.
Answer:
When cyclohexane carbaldehyde reacts with in presence of dry ether and then
(hydrolysis)
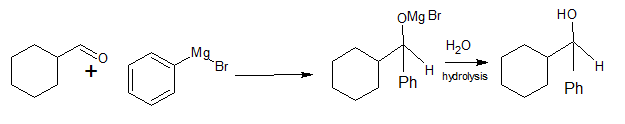
Q 12.6 (ii) Predict the products formed when cyclohexane carbaldehyde reacts with following reagents.
Answer:
Structure of cyclohexanecarbaldehyde
Cyclohexanecarbaldehyde reacts with tollen's reagent and reduce it to silver ( ) and oxidises itself to cyclohexane carboxylate ion
So the reaction is-
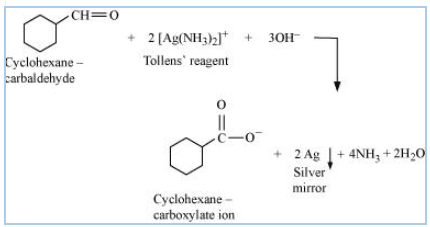
![]()
Q 12.6 (iii) Predict the products formed when cyclohexane carbaldehyde reacts with following reagents.
Answer:
Structure of cyclohexanecarbaldehyde

The reaction of cyclohexane carbaldehyde with semicarbazide and weak acid is-
The group which is not involving in resonance with carbonyl group act as a nucleophile and attack on -CHO group to form product.

Q 12.6 (iv) Predict the products formed when cyclohexane carbaldehyde reacts with following reagents.
Answer:
Structure of cyclohexanecarbaldehyde

reaction of cyclohexanecarbaldehyde with the excess of ethanol and acid to form cyclohexanecarbaldehyde diethyl acetal.

Q 12.6 (v) Predict the products formed when cyclohexane carbaldehyde reacts with following reagents.
Zinc amalgam and dilute hydrochloric acid
Answer:
Structure of cyclohexane carbaldehyde

When cyclohexane carbaldehyde reacts with zinc amalgam( ) and dil.
, the carbonyl group of reactant is reduced to
, it is also known as clemmensen reduction.

Q 12.7 (i) Which of the following compounds would undergo aldol condensation, which the Cannizzaro reaction and which neither? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
Methanal
Answer:
The compounds of ketones or aldehyde having at least one - hydrogen atom give aldol condensation reaction. Here, methanal has not any alpha-hydrogen atom. So, it gives a cannizzaro reaction.
The product of the reactions are, in which in the product is reduced and another is oxidised to carboxylic acid salt-

Answer:
In 2-methylpentanal, there is one alpha-hydrogen atom so it gives aldol condensation reaction. Hence aldol product is-

Answer:
Benzaldehyde Structure

In this structure we can clearly see that there is no alpha-hydrogen atom. So, it gives cannizzaro reaction.

Answer:
It does not give any of the reaction because it is a keto compound and having no alpha -hydrogen atom. Hence it does not give cannizzaro as well as aldol reaction.
Structure -

Answer:
Yes, this compound will give aldol condensation reaction due to the presence of alpha-hydrogen atom. Two moles of cyclohexanone react with each other in which one molecule act as nucleophile and other act as an electrophile.

Answer:
1-Phenylpropanone, has two alpha-hyrdrogen atom, So it gives aldol condensation reaction. It react with an another molecule of 1-Phenylpropanone in presence of dil. .

Answer:
Phenylacetaldehyde, it has two alpha-hydrogen, which makes it possible to perform aldol condensation reaction. So, the reaction is shown here;

Answer:
It does not give any of the reaction, either aldol reaction or cannizzaro reaction because it is a alcohol.
Answer:
In this compound, there is no alpha hydrogen, which means it gives cannizzaro reaction in the presence of conc. sodium hydroxide ( ). One molecule of it is oxidised and the other molecule is reduced.

Q 12.8 (i) How will you convert ethanal into the following compounds?
Answer:
On treating ethanal with dil. alkali it gives 3-hydroxybutanal, which on further reduction with gives the product Butane-1,3-diol

Q 12.8 (ii) How will you convert ethanal into the following compounds?
Answer:
On treating with he presence of dil. alkali ( ), it gives 3-hydroxybuttanal which on further dehydration gives but -2-ene-al.

Q 12.8 (iii) How will you convert ethanal into the following compounds?
Answer:
When the given substrate is reacting with the tollens reagent it produces but-2-enoic acid.

Answer:
Propanal -
butanal -
In cross-aldol condensation, there are total four cases of reaction-
- When two molecules of propanal react with each other
- When two molecules of butanal reacts with eath other
- When propanal act as a nucleophile and attacking on butanal(as an electrophile)
- When butanal act as a nucleophile and attacking on propanal(as an electrophile)
Now the reactions are shown here:
(i)

(ii)

(iii)

(iv)

Answer:
According to given information, the molecular formula is and it reduces tollens reagent. So, there must be an aldehyde group.And also it gives cannizaro reaction, it means the given copound has no
-hydrogen atom. On oxidation it gives 1,2-benzenedicarboxylic acid. Thus, the aldehyde group (
) is directly attached with the benzene ring and also it should be para-substituted.
Hence, the structure of the compound (2-ethyl benzaldehyde) is-

By following reaction we can undrstand this prblem-

Q 12.12 (i) Arrange the following compounds in increasing order of their property as indicated:
Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
Answer:
Increasing order (reactivity towards HCN)-
Di-tert-butyl ketone < Methyl tert-butyl ketone < Acetone < Acetaldehyde

The attacking power of nucleophile can be affected by two ways-
- hindrance near carbonyl group
- negative charge on
group (through +I effect of methyl)
By observing the structure of all compound we can arrange them in order to reactivity towards HCN.
Q 12.12 (ii) Arrange the following compounds in increasing order of their property as indicated:
Answer:
increasing order of their acidic strength-
Due to presence of Bromine, which shows -I effect and we know that -I grows weaker as distance increases. And in case of n-propyl and isopropyl, the +I effect is more in isopropyl, so it is weak in acidic strength.
nature
Q 12.12 (iii) Arrange the following compounds in increasing order of their property as indicated:
Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
Answer:
Increasing order of acidic strength -
4-methoxybenzoic acid < Benzoic acid < 4-Nitrobenzoic acid < 3,4-Dinitrobenzoic acid
As we know, electron withdrawing groups increase the strength of the acid and electron donating group decreases the strength of acids. Therefore, 4-methoxybenzoic acid is the weakest acid among them and 3,4-Dinitrobenzoic acid is the strongest due to the presence of 2 electron withdrawing groups and then 4-Nitrobenzoic acid.
Q 12.13 (i) Give simple chemical tests to distinguish between the following pairs of compounds.
Answer:
Both the copound can be distinguished by following tests-
- tollens test
- iodoform test
- fehlings test
Propanal reduces the tollens reagent but poropanone does not.
Akdehyde responds to fehlings test but ketone does not. So, Propanal gives positive fehlings test but not by propanone.
Q 12.13 (ii) Give simple chemical tests to distinguish between the following pairs of compounds.
Answer:
Acetophenone gives ppositive iodoform test, it react with to give yellow ppt. but benzophenone does not give this test.
benzophenone +
no yellow ppt.
Q 12.13 (iii) Give simple chemical tests to distinguish between the following pairs of compounds.
Answer:
Phenol gives ferric chloride test ( ) , on reaction with ferric chloride phenol gives violet coloured solution but benzoic acid does not.

Q 12.13 (iv) Give simple chemical tests to distinguish between the following pairs of compounds.
Benzoic acid and Ethyl benzoate
Answer:
Both can be distinguish by sodium bicarbonate test, In this test, benzoic acid react with to give brisk effervescence due to evoltion of carbon dioxide (
) but Ethyl benzoate does not.

Q 12.13 (v) Give simple chemical tests to distinguish between the following pairs of compounds.
Answer:
They can be distinguished by iodoform test,
This test is given by compounds having methyl ketone group. So, pentan-2-one give this test and pentan-3-one does not.

Q 12.13 (vi) Give simple chemical tests to distinguish between the following pairs of compounds.
Answer:
Both can be distinguished by-
- Iodoform test
In acetophenone, methyl ketone group present.So it give positive iodoform test by forming a yellow ppt.

- Tollen's test
Aldehyde give positive tollens test. Benzaldehyde reduces the tollens reagent and give red brown ppt.but acetophenone does not give this test.

Q 12.13 (vii) Give simple chemical tests to distinguish between the following pairs of compounds.
Answer:
Ethanal and Propanal can be distinguished by Iodoform test.
Ethanal has one methyl group attached with Carbonyl group, so it gives a positive iodoform test but propanal does not give this test.

Answer:
Benzene on bromination formed bromobenzene and after that we have to react with in presence of ether it becomes Grignard reagents (
). Grignard reagents react with carbon dioxide (dry ice) to form salts of carboxylic acids, which on acidic hydrolysis gives benzoic acid. On esterification, it gives methyl benzoate.

Answer:
Benzene on bromination formed bromobenzene and after that we have to react with in presence of ether it becomes grignard reagents (
). Grignard reagents reacts with carbon dioxide (dry ice) to form salts of carboxylic acids, which on acidic hydrolysis gives benzoic acid. Finally after doing nitration we get our desired product (m-Nitrobenzoic acid).

Answer:
By friedel craft alkylation of benzene give methyl benzene, which on nitration give meta and para substituted methyl benzene, para substituted is major product. Oxidation of para substituted methyl benzene( ) gives salts of carboxylic acid, which on further acidic hydrolysis gives p-nitrobenzoic acid.

Answer:
Alkylation of benzene in presence of anhydrous gives toluene, which on further reaction with
gives benzyl bromide and after that reacts with alc. KCN, CN replace the Br and formed benzyle cyanide which on acidic hydrolysis gives phenyl acetic acid.

Answer:
Alkylationof benzene gives toluene, which on further reaction with gives p- nitrotoluene and react it with the the carbon disulphide /
followed by acidic hydrolysis to form
-nitrobenzaldehyde.

Q 12.15(i) How will you bring about the following conversions in not more than two steps?
Answer:
First, reduce propanone with the help of lithium aluminum hydride ( ) to get propan-2-ol and then by dehydration remove water molecule to get propene.
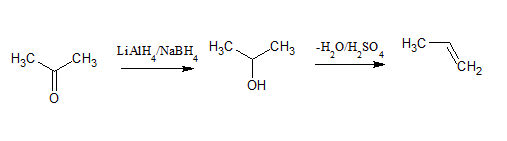
Q 12.15(ii) How will you bring about the following conversions in not more than two steps?
Answer:
With the help of mild reducing agent we can reduce benzoic acids into benzaldehyde.

alternate method-

First react it with which replace OH group and then hydogenation reaction.
Q 12.15 (iii) How will you bring about the following conversions in not more than two steps?
Answer:
Ethanol on heating at 573K in presence of copper it convert into ethanal and then by aldol condensation we get 3-hydroxybutanal.

Q 12.15 (iv) How will you bring about the following conversions in not more than two steps?
Benzene to m-nitroacetophenone
Answer:
Benzene on reacting with acetic anhydride , it gives acetophenone And further reacting acetophenone with nitric acid in presence of sulphuric acid give meta substituted product, m-nitroacetophenone.

Q 12.15 (v) How will you bring about the following conversions in not more than two steps?
Answer:
On oxidation of benzaldehyde, it converted into benzoic acid, which on further reacting with it formed calcium benzoate. After distillation we get benzophenone.

Q 12.15 (vi) How will you bring about the following conversions in not more than two steps?
Bromobenzene to 1-phenylethanol
Answer:
Bromobenzene on reacting with in presence of dry ether it formed grignard reagents (
), which on further reacting with ethanal followe by acidic hydrolysis gives 1-phenyl ethanol

12.15 (vii) How will you bring about the following conversions in not more than two steps?
Benzaldehyde to 3-Phenylpropan-1-ol
Answer:
BY cross aldol-condesation of benzaldehyde and acetaldehyde gives 3-phenylprop-2-ene-al, whixh on catalytic hydrogenation gives 3-Phenylpropan-1-ol

Q 12.15 (viii) How will you bring about the following conversions in not more than two steps?
Benzaldehyde to α-hydroxy phenyl acetic acid
Answer:
Nucleophilic reaction of benzaldehyde with in presence of HCl gives benzaldehyde cyanohydrine, which on acidic hydrolysis formed α-Hydroxyphenylacetic acid

Q 12.15(ix) How will you bring about the following conversions in not more than two steps?
Benzoic acid to m-nitrobenzyl alcohol
Answer:
On nitration of benzoic acid gives meta substituted nitro benzoic acid which on further reacting with reducing agent and followed by acidic hydrolysis gives nitrobenzyl alcohol.

Q 12.16 (i) Describe the following.
Answer:
Acetylation-
The addition of acetyl functional group ( ) in an organic compound is called acetylation. Acetic anhydride(
) and acetyl chloride (
) are mostly used as acetylating agents. This reaction is happens in presence of a base such as pyridine etc.

Q 12.16 (ii) Describe the following.
Answer:
Cannizzaro reaction-
Aldehyde which do not have any alpha- hydrogen, undergo self oxidation as well as reduction reaction on treating with concentrated alkalies. In this reaction, one molecule gets reduced and other is oxidised to carboxylic acid salt.

Q 12.16 (iii) Describe the following.
Answer:
Cross aldol condensation -
In aldol condensation, if the reactants are two different aldehyde or ketone, then it is called cross-aldol condensation. If both of the reactants contains alpha-hydrogen atoms then it gives a mixture of four products.

If the reactants contain alpha-hydrogen atoms then,

Q 12.16 (iv) Describe the following.
Answer:
Decarboxylation -
The reaction in which the carboxylic acid loses carbon dioxide to hydrocarbon, in the presence of sodalime( and
in the ratio of 3:1). This reaction is known as decarboxylation.

Q 12.17(i) Complete each synthesis by giving missing starting material, reagent or products.
Answer:
Potassium permanganate oxidise the substituted ethyl group to group and due to presence of KOH, formed carboxylic acid salts (potassium benzoate)

Q 12.17(ii) Complete each synthesis by giving missing starting material, reagent or products.
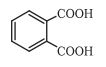
Answer:
The -OH groups of both carboxylic acid is replaced by the chlorine
So, the overall reaction is

Q 12.17 (iii) Complete each synthesis by giving missing starting material, reagent or products
Answer:
In this reaction, the group which not directly attached with the carbonyl group act as a nucleophile and attack on -CO group of benzaldehyde and water molecule is also produced as a by-product.
So, the final product is-

Q 12.17(iv) Complete each synthesis by giving missing starting material, reagent or products.
Answer:
Benzene reacts with benzoyl chloride in presence of anhydrous aluminium chloride ( ) to give benzophenone and also HCl as a by-product.

Q 12.17(v) Complete each synthesis by giving missing starting material, reagent or products.
Answer:
SInce, the give reactant is a aldehyde compound, So it give positive tollen's test by reducing the tollens reagent.

Q 12.17 (vi) Complete each synthesis by giving missing starting material, reagent or products.
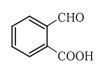
Answer:
In this reaction, the nucleophile CN attacks on -CHO group because it has more reactivity towards .
So the reaction gives

Q 12.17 (vii) Complete each synthesis by giving missing starting material, reagent or products.
Answer:
The given eaction is cross aldol reaction between benzaldehyde and propanal in presence of dil. sodium hydroxide

Q 12.17 (viii) Complete each synthesis by giving missing starting material, reagent or products.
Answer:
In this reaction is a reducing agent, which reduces the CO group of the compound.

Q 12.17(ix) Complete each synthesis by giving missing starting material, reagent or products.
Answer:
oxidises the OH(alcohol) group into keto-group (-CO) and finally the product produced is cyclohexanone

Q 12.17(x) Complete each synthesis by giving missing starting material, reagent or products.
![]()
Answer:
Diborane reacts with an alkene to give trialkyl boranes (addition product) and this is oxidised to alcohol by using hydrogen peroxide.
After then pyridinium chlorochromate (PCC) is reacted with alcohol to convert it into Aldehyde group.

Q 12.17 (xi) Complete each synthesis by giving missing starting material, reagent or products.
Answer:
The above reaction is ozonolysis reaction in which ozone molecule breaks the double bond of the alkene and formed a ketone or aldehyde as a product.

Q 12.18(i) Give plausible explanation for each of the following:
Cyclohexanone forms cyanohydrin in good yield but 2,2,6-trimethylcyclohexanone does not.
Answer:
Structure of 2,2,6-trimethylcyclo hexanone

Cyclohexanone forms cyanohydrin when the nucleophile ( ) attack on cyclohexanone.

In this case, there is less hindrance around the CO group so the nucleophile can attack easily. But in case of 2,2,6-trimethylcyclo hexanone at the alpha ( ) position, there is a hindrance because of the methyl groups. Thus the nucleophile cannot attack effectively.
Q 12.18(ii) Give plausible explanation for each of the following:
Answer:
In Semicarbazide,

One of the group is involved in resonance with the carbonyl group, which is directly attached. Therefore, it cannot act as a nucleophile (its electrons are less available or less electron density at that
group). But the other
group is not participating in resonance, so it can act as nucleophile and attack on the carbonyl carbon of ketone and aldehyde.
Q 12.18(iii) Give plausible explanation for each of the following:
Answer:
If either water or the ester is not removed immediately then ester can react with water and again and give back the reactants.
Thus to shift the reaction in forward direction we need to produce more ester or remove either of the two.
Answer:
In the question, it is given that,
% of carbon = 69.77
% of Hydrogen = 11.63
% of Oxygen = (100-69.77-11.63) = 18.6
Now, the no. of moles of the components are-
Thus ration of C:H:O = 5.81:11.63:1.16
To get empirical formula we need to divide them by 1.16 .So,
C:H:O= 5:10:1
So, the empirical formula is
The molecular mass of the compound =
Since the compound does not give tollens test it means it is not an aldehyde. It is given that, the compound gives positive iodoform test it means the compound must be methyl ketone.
On oxidation, it gives ethanoic acid and propanoic acids. Therefore, the compound should be
Pentan-2-one
the structure of the compound is -
Answer:
In carboxylate ion the electron is delocalised between oxygen, which is electronegative in nature. But in case of phenoxide ion, the electron are delocalise between less electronegative atom and also phenoxide ion has non-equivalent resonance structure. Therefore, carboxylate ion is more resonance stable than phenoxide ion and we know that more stable the conjugate base of an acid, high strong is the acidic

NCERT solutions Class 12 Chemistry Aldehydes Ketones and Carboxylic Acids
Learn here More About Adehyde Ketone and Carboxylic Acid NCERT solutions:
The carbonyl functional group(>C=O) is bonded to a carbon and hydrogen atom, while in the ketones, the carbonyl group is bound to two carbon atoms. In the carboxylic acid, carbon of carbonyl group(>C=O) is bounded to hydrogen or carbon and oxygen of hydroxyl moiety(-OH), while in amides carbon is attached to hydrogen or carbon and nitrogen of moiety and in acyl halides, carbon is attached to hydrogen or carbon and to halogens. Read further to know more about this NCERT solutions for Class 12 Chemistry Chapter 12 PDF download.
In aldehydes, the carbonyl functional group(>C=O) is bonded to a carbon and hydrogen atom, while in the ketones, the carbonyl group is bounded to two carbon atoms. In the carboxylic acid, carbon of carbonyl group(>C=O) is bounded to hydrogen or carbon and oxygen of hydroxyl moiety(-OH), while in amides carbon is attached to hydrogen or carbon and nitrogen of moiety and in acyl halides, carbon is attached to hydrogen or carbon and to halogens. Following are the basic structures of aldehydes, ketones, carboxylic Acids, acyl halide and amide.
An Insight to Aldehydes Ketones and Carboxylic Acids NCERT Solutions:
NCERT Class 12 Chemistry solutions chapter 12 deals with aldehydes, ketones and carboxylic acid compounds, their IUPAC names, physical properties and chemical reactions. In this chapter, there are 20 questions in the exercise. The NCERT solutions for class 12 chemistry chapter 12 Aldehydes, Ketones and Carboxylic Acids will help you in your preparation of CBSE board exams as well as competitive exams like JEE Mains, NEET, BITSAT, etc. In Class 12 Chemistry Chapter 12 NCERT solutions, students have studied organic compounds containing carbon-oxygen single bond functional groups. In NCERT solutions for class 12 chemistry chapter 12 Aldehydes, Ketones and Carboxylic Acids, students will learn about organic compounds containing carbon-oxygen double bond (>C=O) that is known as carbonyl group. In organic chemistry, carbonyl group (>C=O) is one of the most important functional groups.
NCERT Solutions for class 12 Chemistry
NCERT Solutions for class 12 Subject wise
Benefits of NCERT solutions for class 12 chemistry chapter 12 Aldehydes, Ketones and Carboxylic acids
The comprehensive answers given in the NCERT solutions for class 12 chemistry chapter 12 Aldehydes, Ketones and Carboxylic Acids will be helpful in understanding the chapter easily.
Revision will be quite easier because the detailed solutions will help you to remember the concepts and get very good marks in your examination.
Homework problems won't bother you anymore, all you need to do is check the detailed CBSE NCERT solutions for class 12 chemistry chapter 12 Aldehydes, Ketones and Carboxylic Acids and you are good to go.
Also check NCERT Exemplar Class 12 Solutions
- NCERT Exemplar Class 12th Maths Solutions
- NCERT Exemplar Class 12th Physics Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Class 12th Biology Solutions
Also Check NCERT Books and NCERT Syllabus here:
- NCERT Books Class 12 Chemistry
- NCERT Syllabus Class 12 Chemistry
- NCERT Books Class 12
- NCERT Syllabus Class 12
Frequently Asked Question (FAQs)
For complete solutions : https://school.careers360.com/ncert/ncert-solutions-class-12-chemistry
Aldehydes
Ketones
Hydration of Alkynes
Formation of Alcohols
Wolff- Kishner Reduction
Halogenation
Cannizzaro Reaction
Friedel- Crafts Acylation.
Nuclei Addition Reactions
Formation of Cyanohydrins
Weightage of this chapter is 3 percent. The questions of NEET can be practiced using NCERT book exercises, NCERT exemplar pronlems and NEET previous year papers.
This chapter holds weightage of 2-3 marks in JEE mains
Weightage of this chapter in CBSE Board exams is 5-6 marks.
Also Read
Articles
Certifications By Top Providers
Explore Top Universities Across Globe
Questions related to CBSE Class 12th
Hello aspirant,
The purpose of graphic design extends beyond the brand's look. Nevertheless, by conveying what the brand stands for, it significantly aids in the development of a sense of understanding between a company and its audience. The future in the field of graphic designing is very promising.
There are various courses available for graphic designing. To know more information about these courses and much more details, you can visit our website by clicking on the link given below.
https://www.careers360.com/courses/graphic-designing-course
Thank you
Hope this information helps you.
hello,
Yes you can appear for the compartment paper again since CBSE gives three chances to a candidate to clear his/her exams so you still have two more attempts. However, you can appear for your improvement paper for all subjects but you cannot appear for the ones in which you have failed.
I hope this was helpful!
Good Luck
Hello dear,
If you was not able to clear 1st compartment and now you giving second compartment so YES, you can go for your improvement exam next year but if a student receives an improvement, they are given the opportunity to retake the boards as a private candidate the following year, but there are some requirements. First, the student must pass all of their subjects; if they received a compartment in any subject, they must then pass the compartment exam before being eligible for the improvement.
As you can registered yourself as private candidate for giving your improvement exam of 12 standard CBSE(Central Board of Secondary Education).For that you have to wait for a whole year which is bit difficult for you.
Positive side of waiting for whole year is you have a whole year to preparing yourself for your examination. You have no distraction or something which may causes your failure in the exams. In whole year you have to stay focused on your 12 standard examination for doing well in it. By this you get a highest marks as a comparison of others.
Believe in Yourself! You can make anything happen
All the very best.
Hello Student,
I appreciate your Interest in education. See the improvement is not restricted to one subject or multiple subjects and we cannot say if improvement in one subject in one year leads to improvement in more subjects in coming year.
You just need to have a revision of all subjects what you have completed in the school. have a revision and practice of subjects and concepts helps you better.
All the best.
Hi,
You just need to give the exams for the concerned two subjects in which you have got RT. There is no need to give exam for all of your subjects, you can just fill the form for the two subjects only.
Popular CBSE Class 12th Questions
Colleges After 12th
Also Read
Applications for Admissions are open.

JEE Main Important Physics formulas
ApplyAs per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

UPES School of Liberal Studies
ApplyRanked #52 Among Universities in India by NIRF | Up to 30% Merit-based Scholarships | Lifetime placement assistance

Aakash iACST Scholarship Test 2024
ApplyGet up to 90% scholarship on NEET, JEE & Foundation courses

JEE Main Important Chemistry formulas
ApplyAs per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

PACE IIT & Medical, Financial District, Hyd
ApplyEnrol in PACE IIT & Medical, Financial District, Hyd for JEE/NEET preparation

ALLEN JEE Exam Prep
ApplyStart your JEE preparation with ALLEN