Apply to Aakash iACST Scholarship Test 2024
NCERT solutions for class 12 chemistry chapter 9 Coordination Compounds
NCERT Solutions for Class 12 Chemistry Chapter 9 Coordination Compounds - Coordination compounds is a conceptual chapter related to chemical reactions and atoms so you must pay special attention to the NCERT solutions for Class 12 Chemistry chapter 9 Coordination Compounds which are provided here for free. Coordination compounds are an important part of inorganic chemistry and coordination compounds. As per the NCERT, coordination compounds are the foundation of modern Inorganic Chemistry. In this NCERT solution, there are 11 in-text questions and 32 questions in the exercise and you will find all the NCERT Solutions for Class 12 Chemistry Chapter 9 when you scroll down.
Latest: JEE Main high scoring chapters
Don't Miss: JEE Main 10 year's papers
Recommended: NEET high scoring chapters
Suggested: NEET question papers with solutions

Also Read,
- NCERT Exemplar Solutions For Class 12 Chemistry Chapter 9 Coordination Compounds
- NCERT Notes For Class 12 Chemistry Chapter 9 Coordination Compounds
Find NCERT Solutions for Class 12 Chemistry Chapter 9 Coordination Compounds Below:
Solutions to In-Text Questions Ex 9.1 to 9.10
Question 9.1(i) Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt(III) chloride
Answer :
The chemical formula for the coordination compound Tetraamminediaquacobalt(III) chloride is
Question 9.1(ii) Write the formulas for the following coordination compounds:
(ii) Potassium tetracyanidonickelate(II)
Answer :
The formula for the coordination compound Potassium tetracyanidonickelate II is :
Question 9.1(iii) Write the formulas for the following coordination compounds:
(iii) Tris(ethane–1,2–diamine) chromium(III) chloride
Answer :
The formula for the coordination compound Tris(ethane–1,2–diamine) chromium(III) chloride is :
Question 9.1(iv) Write the formulas for the following coordination compounds:
(iv) Amminebromidochloridonitrito-N-platinate(II)
Answer :
The formula for the coordination compound Amminebromidochloridonitrito-N-platinate(II) :
Question 9.1(v) Write the formulas for the following coordination compounds:
(v) Dichloridobis(ethane–1,2–diamine)platinum(IV) nitrate
Answer :
The chemical formula for the coordination compounds: Dichloridobis(ethane–1,2–diamine)platinum(IV) nitrate
Question 9.1(vi) Write the formulas for the following coordination compounds:
(vi) Iron(III) hexacyanidoferrate(II)
Answer :
The formula for the coordination compound Iron(III) hexacyanidoferrate(II):
Question 9.2(i) Write the IUPAC names of the following coordination compounds:
Answer :
The IUPAC name of the coordination compound is :
Hexaamminecobalt(III) chloride
Question 9.2(ii) Write the IUPAC names of the following coordination compounds:
Answer :
The IUPAC name of the coordination compound is
Pentaamminechloridocobalt(III) chloride
Question 9.2(iii) Write the IUPAC names of the following coordination compounds:
Answer :
The IUPAC name of the coordination compound is
Potassium hexacyanoferrate(III).
Question 9.2(iv) Write the IUPAC names of the following coordination compounds:
Answer :
The IUPAC name of the following coordination compound is
Potassium trioxalatoferrate(III)
Question 9.2(v) Write the IUPAC names of the following coordination compounds:
Answer :
The IUPAC names of the coordination compound is
Potassium tetrachloridopalladate(II).
Question 9.2(vi) Write the IUPAC names of the following coordination compounds:
Answer :
The IUPAC name of the following coordination compound is :
Diamminechlorido(methylamine)platinum(II) chloride.
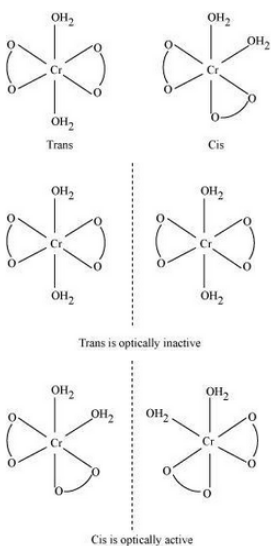
Question 9.3(i) Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
Answer :
Both geometrical (cis-, trans-) and optical isomers exists for the given compound.
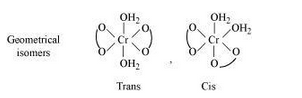
Question 9.3(ii) Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
Answer :
Two optical isomers can exist, Dextro and Laevo.
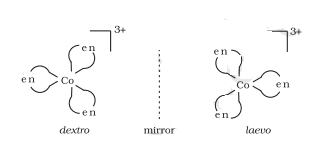
Question 9.3(iii) Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
Answer :
There are 10 possible isomers.There are geometrical, ionisation and linkage isomers possible.
A pair of optical isomer
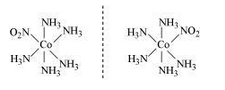
it also shows ionization isomerism
AND
It can also show linkage isomerism
AND
Question 9.3(iv) Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
Answer :
Geometrical (cis-, trans-) isomers can exists for
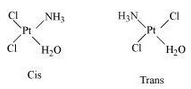
Question 9.4(i) Give evidence that following is ionisation isomer.
Answer :
The ionisation isomers dissolve in water to yield different ions and thus react differently to various reagents:
[Co(NH3)5Br]SO4 + Ba2+ → BaSO4 (s)
[Co(NH3)5SO4]Br + Ba2+ → No reaction
[Co(NH3)5Br]SO4 + Ag+ → No reaction
[Co(NH3)5SO4]Br + Ag+ → AgBr (s)
Question 9.4(ii) Give evidence that following is ionisation isomer.
Answer :
The ionisation isomers dissolve in water to yield different ions and thus react differently to various reagents:
[Co(NH3)5Br]SO4 + Ba2+ → BaSO4 (s)
[Co(NH3)5SO4]Br + Ba2+ → No reaction
[Co(NH3)5Br]SO4 + Ag+ → No reaction
[Co(NH3)5SO4]Br + Ag+ → AgBr (s)
Answer :
In both the compounds, the oxidation state of Nickel is +2. So it has d 8 configuration.
Now, on the basis of ligand pairing of electrons will occur. Since CN - is a strong ligand so pairing will occur but in case of Cl - pairing will not be there as it is a weak ligand. So, the configuration of both the compounds looks like :-
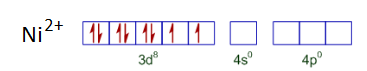
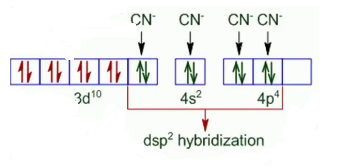
Thus is a square planer and diamagnetic and
has tetrahedral geometry and is paramagnetic.
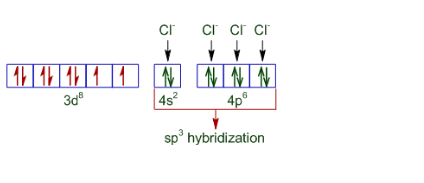
Question 9.6 is paramagnetic while
is diamagnetic though both are tetrahedral. Why?
Answer :
The difference in the magnetic behaviour is due to the nature of ligands present. In case of the oxidation state of nickel is +2 and also Cl - is a weak ligand. Thus its configuration becomes:-
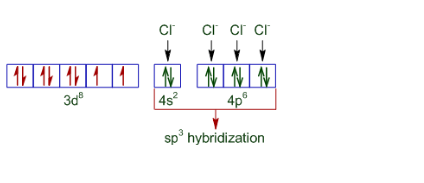
So it is paramagnetic and tetrahedral in nature.
In the case of , the oxidation state of nickel is 0. So its configuration is 3d 8 4s 2 . We also know that CO is a strong ligand, thus the configuration of nickel becomes:-
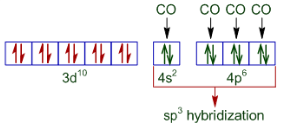
Hence the given compound is diamagnetic but tetrahedral in nature.
Question 9.7 is strongly paramagnetic whereas
is weakly paramagnetic. Explain.
Answer :
In both the compounds Fe has +3 oxidation state i.e., d 5 configuration.
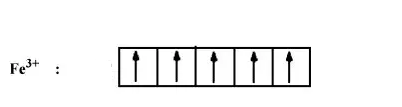
In the case of strong ligand (CN - ), the pairing of electron will occur. So number of electrons left unpaired will be 1.
In case of weak ligand (H 2 O), pairing of electron will not there. Thus number of electrons unpaired will be 5.
We know that paramagnetic strength is directly proportional to the number of unpaired electrons.
Hence paramagnetism will be more in case of
Question 9.8 Explain is an inner orbital complex whereas
is an outer orbital complex.
Answer :
Firstly consider :-
The oxidation state of cobalt is +3. So the electronic configuration of it will be d 6 .
Since (NH 3 ) is a strong ligand so the pairing of electron will be there.
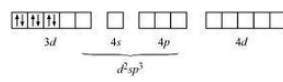
So, it has d 2 sp 3 hybridisation and an inner orbital complex.
Now consider,
The oxidation state of nickel is +3. So its electronic configuration will be d 8 .
Also (NH 3 ) is a strong ligand so pairing of electrons will be seen.
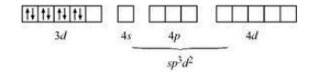
Thus is an outer orbital complex.
Question 9.9 Predict the number of unpaired electrons in the square planar ion.
Answer :
The oxidation state of Pt in the given compound is +2. Also, it is given that the compound has square planar geometry i.e., it has dsp 2 hybridisation (d 8 ).
CN - is a strong ligand so the pairing of electron will occur.
So there are be no unpaired electrons in the given compound.
Answer :
Consider Hexaqua manganese (II) :- In this compound, the oxidation state of Mn is +2 and its electronic configuration is d 5 .
H 2 O is a weak ligand and crystal field is octahedral so the arrangement of electrons will be t 2 3 g eg 2 .
So the total number of unpaired electrons is 5.
Now consider hexacyanoion:- In this compound, the oxidation state of Mn is +2. It is surrounded by the strong ligands CN - , so pairing will be there.
Its arrangement will be t 2g 5 eg 0 .
Thus the number of unpaired electrons will be 1.
NCERT Solutions for Class 12 Chemistry Chapter 9 Coordination Compounds- Exercise Questions
Question 9.1 Explain the bonding in coordination compounds in terms of Werner’s postulates.
Answer :
Werner his theory of coordination compounds and gave some postulates. The main postulates are:
1. In coordination compounds metals show two types of linkages or valences namely primary valency and secondary valency.
2. The primary valences are generally ionisable and are satisfied or balanced by negative ions.
3. The secondary valences are non-ionisable. These are satisfied by either neutral molecules or by negative ions. The secondary valence is equal to the coordination number (No. of atoms surrounding the metal) and is constant for a metal.
4. According to different coordination numbers, the ions/groups bound by the secondary linkages to the metal have characteristic spatial arrangements.
Answer :

![]()
The major difference between both the compounds is that the first compound is a salt and the other one is a coordination compound. In case of double salt compounds (Mohr's salt), the compound breaks into its constituent ions when dissolved in water, therefore it gives a positive test for the presence of Fe +2 . But in case of coordination compounds, they maintain their identity in both solid and dissolved state. Thus the individual property of each constituent is lost. And therefore it doesn't give a positive test for Cu +2 .
Answer :
(i) Coordination entity:- It is an electrically charged species carrying either a positive charge or a negative charge. In a coordination entity, the central atom or ion is surrounded by some number of neutral molecules or negative ions ( called ligands) accordingly.
(ii) Ligand:- Ligands are the neutral molecules or negatively charged ions that surround the metal atom in a coordination entity according to the holding capacity of central metal ion are known as ligands. NH 3 and H 2 O are two neutral ligands.
(iii) Coordination number:- The total number of metals that surrounds the central metal ion is known as coordination number.
For e.g,(a) In case of six chlorine atoms are attached to Pt, thus the coordination number of the given compound is 6.
(b) In case of the central metal ion Ni is surrounded by 4 atoms of ligand so its coordination number is 4.
(iv) Coordination polyhedron:- It is defined as the spatial arrangement of the ligands which are directly attached to the central metal ion/atom.
E.g. square planar, tetrahedral.
(v) Homoleptic:- Homoleptic compounds are defined as the compounds in which the donor/ligand attached to central metal atom/ion is of one kind.
E.g. and
(vi) Heteroleptic:- These are the coordination compounds in which central atoms are attached with more than one type of ligand.
E.g. and
Question 9.4 What is meant by unidentate, didentate and ambidentate ligands? Give two examples for each.
Answer :
Unidentate ligands:- The ligand which has only one donor site are known as unidentate ligands. E.g. Cl - , NH 3
Bidentate(or didentate):- The ligands which have two donor sites are known as bidentate ligands. E.g. Ethane - 1,2 diamine, Oxalate ion
Ambidentate ligands:- The ligands which can attach themselves to the central metal through two different atoms. E.g NO 2 , SCN
Question 9.5 (i) Specify the oxidation numbers of the metals in the following coordination entities:
Answer :
Let us assume that coordination number of cobalt is x.
Thus
Hence coordination number of cobalt is +3.
Question 9.5(ii) Specify the oxidation numbers of the metals in the following coordination entities:
Answer :
Let us assume that the coordination number of cobalt is x.
Then according to the question :
Hence the coordination number of cobalt is 3.
Question 9.5(iii) Specify the oxidation numbers of the metals in the following coordination entities:
Answer :
Let us assume the oxidation state of Pt to be x.
Then the equation becomes :-
Hence the oxidation state of Pt metal is 2.
Question 9.5(iv) Specify the oxidation numbers of the metals in the following coordination entities:
Answer :
Let the oxidation state of Fe be x.
Then the equation will be :-
Thus oxidation state of Fe in this coordination compound is 3.
Question 9.5(v) Specify the oxidation numbers of the metals in the following coordination entities:
Answer :
Let us assume the oxidation state of Cr to be x.
Then according to the question we get,
So the oxidation state of Cr will be 3.
Question 9.6(i) Using IUPAC norms write the formulas for the following:
(i) Tetrahydroxidozincate(II)
Answer :
Using the IUPAC rules :-
Question 9.6(ii) Using IUPAC norms write the formulas for the following:
(ii) Potassium tetrachloridopalladate(II)
Answer :
The required compound is :-
Question 9.6(iii) Using IUPAC norms write the formulas for the following:
(iii) Diamminedichloridoplatinum(II)
Answer :
The required chemical formula of compound is :-
Question 9.6(iv) Using IUPAC norms write the formulas for the following:
(iv) Potassium tetracyanidonickelate(II)
Answer :
The required compound is :-
Question 9.6(v) Using IUPAC norms write the formulas for the following:
(v) Pentaamminenitrito-O-cobalt(III)
Answer :
The compound is :-
Question 9.6(vi) Using IUPAC norms write the formulas for the following:
(vi) Hexaamminecobalt(III) sulphate
Answer :
The required compound is :-
Question 9.6(vii) Using IUPAC norms write the formulas for the following:
(vii) Potassium tri(oxalato)chromate(III)
Answer :
The required compound is :-
Question 9.6(viii) Using IUPAC norms write the formulas for the following:
(viii) Hexaammineplatinum(IV)
Answer :
The required compound is :-
Question 9.6(ix) Using IUPAC norms write the formulas for the following:
(ix) Tetrabromidocuprate(II)
Answer :
The required compound is
Question 9.6(x) Using IUPAC norms write the formulas for the following:
(x) Pentaamminenitrito-N-cobalt(III)
Answer :
The required compound is :-
Question 9.7(i) Using IUPAC norms write the systematic names of the following:
Answer :
Using IUPAC norms the name of the given compound is :-
Hexaamminecobalt(III) chloride
Question 9.7(ii) Using IUPAC norms write the systematic names of the following:
Answer :
According to the IUPAC norms :-
Diamminechlorido(methylamine) platinum(II) chloride
Question 9.7(iii) Using IUPAC norms write the systematic names of the following:
Answer :
Using the nomenclature rules, the required name of the compound is:- Hexaquatitanium(III) ion
Question 9.7(iv) Using IUPAC norms write the systematic names of the following:
Answer :
Using IUPAC norms, the name of the given compound is :-
Tetraamminichloridonitrito-N-Cobalt(III) chloride
Question 9.7(v) Using IUPAC norms write the systematic names of the following:
Answer :
The IUPAC name of the givne compound is :-
Hexaquamanganese(II) ion
Question : 9.7(vi) Using IUPAC norms write the systematic names of the following:
Answer :
According to the IUPAC norms, the systematic name of the given compound is:- Tetrachloridonickelate(II) ion
Question 9.7(vii) Using IUPAC norms write the systematic names of the following:
Answer :
The IUPAC name of the given compound is :- Hexaamminenickel(II) chloride
Question 9.7(viii) Using IUPAC norms write the systematic names of the following:
Answer :
The name of the given compound is :- Tris(ethane-1, 2-diammine) cobalt(III) ion
Question 9.7(ix) Using IUPAC norms write the systematic names of the following:
Answer :
The IUPAC name of the given compound is :- Tetracarbonylnickel(0)
Question 9.8 List various types of isomerism possible for coordination compounds, giving an example of each.
Answer :
Two main types of isomerism are known in case of coordination compounds which can be further divided into subgroups:-
(a) Stereoisomerism :-
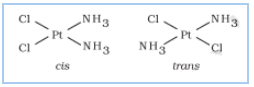
(i) Geometrical isomerism E.g
. 
![]()
(ii) Optical isomerism
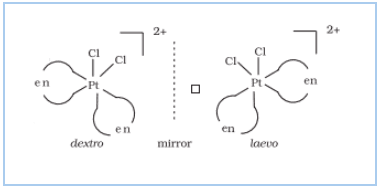
![]()
(b) Structural isomerism :-
(i) Linkage isomerism E.g and
(ii) Ionisation isomerism E.g and
(iii) Coordination isomerism E.g and
(iv) Solvate isomerism E.g and
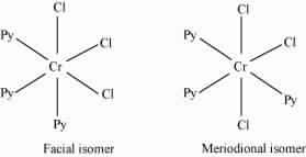
Question : 9.9(i) How many geometrical isomers are possible in the following coordination entities?
No geometrical isomers are possible since the given compound is a bidentate compound.
Question 9.9(ii) How many geometrical isomers are possible in the following coordination entities?
Answer :
The facial (fac) and meridional (mer) isomers are possible for the given compound.
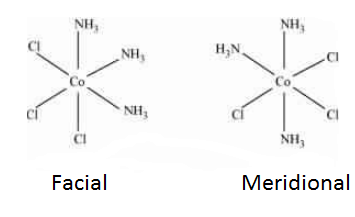
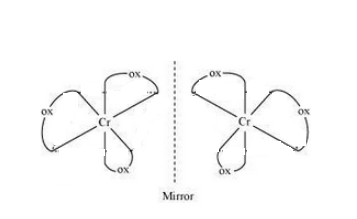
Question 9.10(i) Draw the structures of optical isomers of:
Answer :
The optical isomers of the given compound are:-
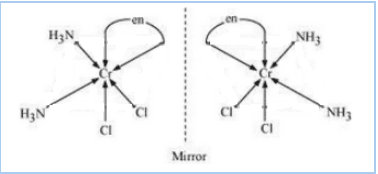
Question 9.10(ii) Draw the structures of optical isomers of:
Answer :
The optical isomers of the given compound are given below :-
![]()
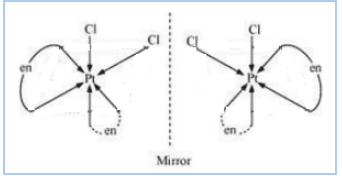
Question 9.10(iii) Draw the structures of optical isomers of:
Answer :
The optical isomers of the given compound are:-
![]()
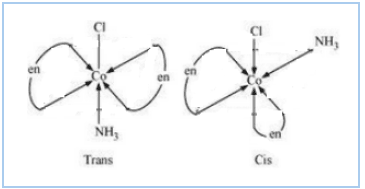
Question 9.11(i) Draw all the isomers (geometrical and optical) of:
Answer :
The configurational isomers are:-
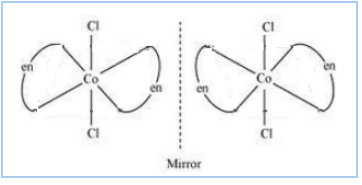
![]()
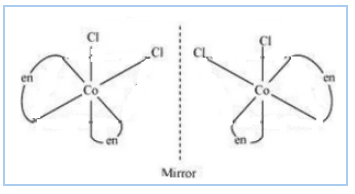
![]()
Question 9.11(ii) Draw all the isomers (geometrical and optical) of:
Answer :
The isomers of the given compound are :-
![]()
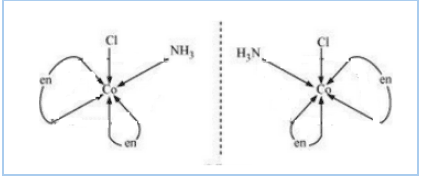
![]()
Question 9.11(iii) Draw all the isomers (geometrical and optical) of:
Answer :
The possible isomers of the given compound are as follows :-
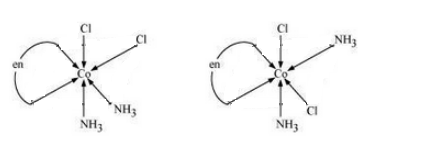
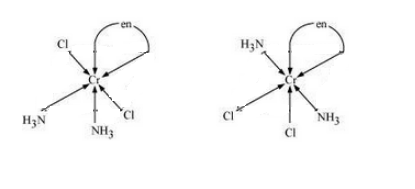
Question 9.12 Write all the geometrical isomers of and how many of these will exhibit optical isomers?
Answer :
The geometrical isomers of the compound are given below:-
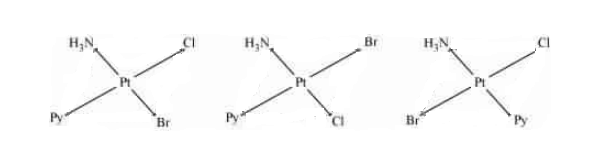
We know that the given compound has tetrahedral geometry, so it can be optically active only when it has unsymmetric chelating agents. Hence the given compound doesn't have any optically active isomer.
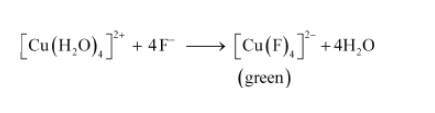
Question 9.13 Aqueous copper sulphate solution (blue in colour) gives:
(i) a green precipitate with aqueous potassium fluoride and
(ii) a bright green solution with aqueous potassium chloride. Explain these experimental results.
Answer :
We know that strong ligands can replace weak ligands from its solution.
(i) In this case F - ions can replace H 2 O from aqueous copper sulphate solution.
(ii) In this compound, Cl - being stronger ligand will replace H 2 O and give a bright green solution 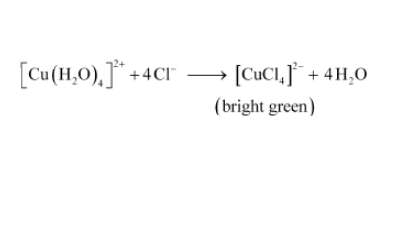
Answer :
When KCN is passed through an aqueous solution of copper sulphate, CN - being a strong ligand, will replace water and form .
It is known that in stable coordination compounds, the individual identity of each constituent is lost i.e., Cu +2 is not available freely.
Thus no precipitate of copper sulphide is obtained in the given conditions.
Question 9.15 Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
Answer :
In the given compound oxidation state of Fe is +2. The electronic configuration of this compound is 3d 6 .
Also CN - is a strong field ligand so it will cause the pairing of electrons.
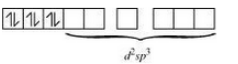
Therefore the complex is diamagnetic and its geometry is octahedral.
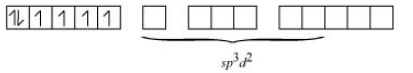
Question 9.15 Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
Answer :
(ii) In the given complex the oxidation state of Fe is +3. Its electronic configuration is d 5 .
Also, the F - ions are weak field ligands, therefore, the pairing of electrons will not occur.
Thus its geometry is octahedral and it is paramagnetic in nature.
Question 9.15 Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
Answer:
(iii) In the given compound, the oxidation state of Co is +3. Its electronic configuration thus becomes d 6 .
Also, oxalate is a weak field ligand therefore pairing of electrons will not occur.
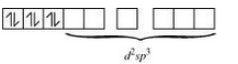
Hence the complex is octahedral and paramgnetic in nature.
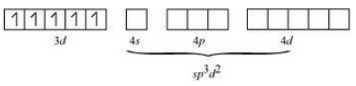
Question 9.15 Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
Answer :
(iv) The oxidation state of Co in the given comound is +3. The electronic configuration of the compound is d 6 .
Also, F - is a weak field ligand so no pairing of electrons will occur.
nbsp;
Hence the geometry of compound is octahedral and it is paramagnetic in nature.
Question 9.16 Draw figure to show the splitting of d orbitals in an octahedral crystal field.
Answer :
The splitting of d orbital is shown below:-
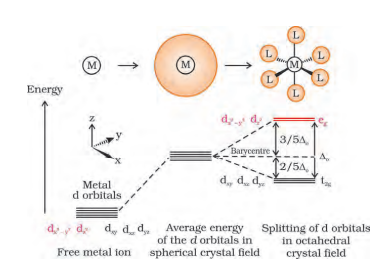
In this splitting d x 2 y 2 and d z 2 experience a rise in energy and make the eg level, while d xy , d yz and d zx experience a fall in energy and generate the t 2g level.
Question 9.17 What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
Answer :
The arrangement of ligands in the increasing order of their crystal-field splitting energy (CFSE) values is known as spectrochemical series .
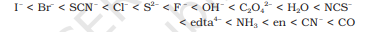
The ligands on the right side of the series strong field ligands are present whereas on the left-hand side weak field ligands are present.
The strong field ligands are capable of splitting d orbitals to a higher extent as compared to weak field ligands.
Answer :
It is known that the degenerated d-orbitals split into two levels - e g and t 2g . The splitting of the degenerate levels due to the presence of ligands is called the crystal-field splitting and the energy difference between the two levels (e g and t 2g ) is called the crystal-field splitting energy (CFSE).
The CFSE is denoted by Δ o .
After splitting of orbitals, the filling of the electrons starts. After 1 electron has been filled in each of the three t 2g orbitals, the fourth electron can enter the eg orbital ( t 2g 3 e g 1 like electronic configuration) or the pairing of the electrons can take place in the t 2g orbitals ( t 2g 4 e g 0 like electronic configuration).
If the CFSE value or Δ o value of a ligand is less than the pairing energy (P), then the electrons enter into the e g orbital. And, if the Δ o value of a ligand is more than the pairing energy (P), then the electrons will enter the t 2g orbital.
Question 9.19 is paramagnetic while
is diamagnetic. Explain why?
Answer :
In the oxidation state of the compound is +3. Its electronic configuration is d 3 . Also, NH 3 is a weak field ligand so the pairing of electrons will not occur.
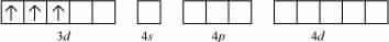
So this compound is paramagnetic in nature.
In the oxidation state of the Ni is +2. Its electronic configuration is d 8 . Also, CN - is a strong field ligand so the pairing of electrons will occur.

Hence the above compound is diamagnetic in nature.
Question 9.20 A solution of is green but a solution of
is colourless. Explain .
Answer :
In the case of , we have CN - as a strong field ligand. So, the pairing of electrons will occur. The electronic configuration of Ni +2 is d 6 . Since all the electrons will be paired thus d-d electronic transition is not possible on this case. Whereas in case of
we have a weak field ligand (H 2 O). The pairing of electrons will not occur. Thus electrons from a lower state of energy can transit to a higher state of energy and thus will give some colour.
Question 9.21 and
are of different colours in dilute solutions. Why?
Answer :
In both compounds, the oxidation state of Fe is +2. Also, in we have weak field ligand whereas in
we have strong field ligand. So there is a difference in CFSE value in both the compounds. As a result, the colour shown by both compounds is different.
Question 9.22 Discuss the nature of bonding in metal carbonyls.
Answer :
The metal-carbon bond in metal carbonyls has both σ and π character. Basically the M–C σ bond is generated due to the donation of lone pair of electrons on the carbonyl carbon into a vacant orbital of the metal. Whereas the M–C π bond is formed due to the donation of a pair of electrons from a filled d orbital of metal into the vacant/empty antibonding π* orbital of carbon monoxide. As a result, this metal to ligand bonding leads to a synergic effect which strengthens the bond between CO and the metal.
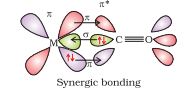
Question 9.23 Give the oxidation state, d orbital occupation and coordination number of the central metal ion in the following complexes:
Answer :
The oxidation state of Co is +3 and its oxidation number is 6. The d orbital occupation of the given central metal ion is t 2g 6 e g 0 .
Question 9.23 Give the oxidation state, d orbital occupation and coordination number of the central metal ion in the following complexes:
Answer :
(ii) The oxidation state of Cr in the given complex is +3. The coordination number of Cr is 6. The d orbital occupation for central metal ion Cr 3+ is t 2g 3 .
Question 9.23 Give the oxidation state, d orbital occupation and coordination number of the central metal ion in the following complexes:
Answer :
(iii) The oxidation state of Co in the given coordination compound is +2. Also, the coordination number of Co is 4. The d orbital occupation for the central metal ion Co 2+ is e g 4 t 2g 3 .
Question 9.23 Give the oxidation state, d orbital occupation and coordination number of the central metal ion in the following complexes:
Answer :
(iv) In the given complex compound the oxidation state of Mn is +2. Also, the coordination number of Mn is 6. The d orbital occupation for the central metal ion Mn +2 is t 2g 3 e g 2 .
Answer :
The IUPAC name of the given compound is Potassium diaquadioxalatochromate (III) trihydrate.
The oxidation state of Cr in this compound is +3.
The electronic configuration is 3d 3 .
The coordination number of this compound is 6.
The magnetic moment of a compound is given by :
Here n is the number of the unpaired electrons.
or
Stereochemistry:-
Answer :
The IUPAC name of the given compound is Pentaamminechloridocobalt(III) chloride.
The oxidation state of Co in this compound is +3.
The electronic configuration is d 6 .
The coordination number of this compound is 6.
The magnetic moment of a compound is given by :
Here n is the number of the unpaired electrons.
or
Stereochemistry:-
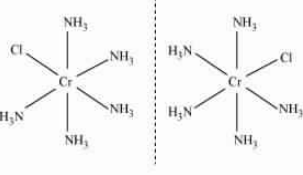
Answer :
The IUPAC name of the given compound is Trichloridotripyridinechromium (III).
The oxidation state of Cr in this compound is +3.
The electronic configuration is d 3 .
The coordination number of this compound is 6.
The magnetic moment of a compound is given by :
Here n is the number of the unpaired electrons.
or
Stereochemistry:-
Answer :
The IUPAC name of the given compound is Caesium tetrachloroferrate (III).
The oxidation state of Cs in this compound is +3.
The electronic configuration is d 5 .
The coordination number of this compound is 4.
The magnetic moment of a compound is given by :
Here n is the number of the unpaired electrons.
or
Stereochemistry:- Optically inactive.
Answer :
The IUPAC name of the given compound is Potassium hexacyanomanganate(II).
The oxidation state of Mn in this compound is +2.
The electronic configuration is d 5 .
The coordination number of this compound is 6.
The magnetic moment of a compound is given by :
Here n is the number of the unpaired electrons.
or
Stereochemistry:- Optically inactive.
Question 9.25 Explain the violet colour of the complex on the basis of crystal field theory.
Answer :
In ground state,Ti has 23 electrons with electronic configuration 3d 3 4s 2 .
The oxidation state of Ti in the given compound is +3.
Hence it will now have the configuration 3d 2 . Since it has 2 unpaired electrons and has the ability to undergo d-d transition, the given complex gives violet colour.
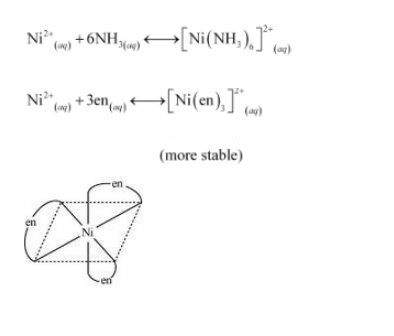
Question 9.26 What is meant by the chelate effect? Give an example .
Answer :
When a ligand is attached to the metal ion in such a manner that it forms a ring-like structure, then the metal-ligand bond is found to be more stable i.e., complexes containing chelate rings are more stable than complexes without rings. The formation of such rings is known as the chelate effect.
Question 9.27 Discuss briefly giving an example in each case the role of coordination compounds in:
(i) biological systems
Answer :
Coordination compounds play a great role in biological systems. The pigment which is responsible for photosynthesis, chlorophyll, is a coordination compound of magnesium. Haemoglobin (which acts as oxygen carrier) the red pigment of blood is a coordination compound of iron. Vitamin B 12 , cyanocobalamine, the anti-pernicious anaemia factor, are few coordination compounds of cobalt which have biological importance.
Question 9.27 Discuss briefly giving an example in each case the role of coordination compounds in:
(ii) medicinal chemistry
Answer :
The role of coordination compounds in the medicine industry is very huge such as the use of chelate therapy in medicinal chemistry. The excess of copper and iron are removed by the chelating ligands D–penicillamine and desferrioxamine B via the formation of coordination compounds. Nowadays, some coordination compounds of platinum (such as cis–platin and related compounds) effectively inhibit the growth of tumours.
Question 9.27 Discuss briefly giving an example in each case the role of coordination compounds in:
(iii) analytical chemistry
Answer :
(iii) In analytical chemistry, the familiar colour reactions given by metal ions with a number of ligands generally chelating ligands. The formation of coordination entities gives the basis for their detection and estimation by classical and instrumental methods of analysis.
Question 9.27 Discuss briefly giving an example in each case the role of coordination compounds in:
(iv) extraction/metallurgy of metals
Answer :
In the metal extraction process of metals, like silver and gold, make use of complex formation. For example, gold combines with cyanide in the presence of oxygen and water to form the coordination entity [Au(CN) 2 ] in aqueous solution which can be further separated by addition of zinc.
Question 9.28 How many ions are produced from the complex in solution?
(i) 6 (ii) 4 (iii) 3 (iv) 2
Answer :
Total of 3 ions will be produced.
2 of Cl - and one ion of [Co(NH 3 ) 6 ] + will be produced.
Question 9.29 Amongst the following ions which one has the highest magnetic moment value?
Answer :
(i) No. of unpaired electrons in is 3.
The magnetic moment is given by :
or
(ii) Similarly in the number of unpaired electrons is 4.
So the magnetic moment :
(iii) In the case of the number of unpaired electrons is 0. So its magnetic moment is also zero.
Thus has the highest dipole moment among all.
Question 9.31 Amongst the following, the most stable complex is
Answer :
We know that due to the chelation effect stability of the chelating compound is more than the simple compound. Thus it is easy to notice that is most stable among all given compounds.
Question 9.32 What will be the correct order for the wavelengths of absorption in the visible region for the following:
Answer :
The order of the wavelength of absorption will be decided from the order of their CFSE values.
The CFSE values increase in the order :- H 2 O < NH 3 < NO 2 -
Hence, the order of wavelengths of absorption in the visible region is : [Ni(H 2 O) 6 ] 2+ > [Ni(NH 3 ) 6 ] 2+ > [Ni(NO 2 ) 6 ] 4-
NCERT Solutions Class 12 Chemistry - Chapterwise
More About NCERT Solutions for Class 12 Chemistry Chapter 9 Coordination Compounds
After studying chapter 9 Coordination Compounds of Class 12 Chemistry, you will be able to know the meaning of terms like a central atom or ion, coordination entity, ligand, coordination number, oxidation number, homoleptic and heteroleptic, able to learn the rules of nomenclature of coordination compounds, define various types of isomerism in coordination compounds and various applications of coordination compounds in daily life.
Topics and Sub-topics of NCERT solutions for Class 12 Chemistry Chapter 9 Coordination Compounds-
9.1 Werner's Theory of Coordination Compounds
9.2 Definitions of Some Important Terms Pertaining to Coordination Compounds
9.3 Nomenclature of Coordination Compounds
9.4 Isomerism in Coordination Compounds
9.5 Bonding in Coordination Compounds
9.6 Bonding in Metal Carbonyls
9.7 Importance and Applications of Coordination Compounds
NCERT Solutions for Class 12 Subject-wise
- NCERT Solutions for Class 12 Biology
- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 12 Physics
Benefits of NCERT solutions for class 12 chemistry chapter 9 Coordination Compounds
The NCERT Class 12 Chemistry solutions chapter 9 Coordination Compounds are prepared and explained well by subject experts to help you in the preparation of CBSE Board exams.
NCERT solutions for other classes and subjects can aid you in various competitive exams like JEE, NEET, BITS, and, KVPY etc.
The seven sub-topics of NCERT syllabus Chemistry chapter 9 Coordination Compounds covers important concepts like Werner’s Theory of coordination compounds, definitions of some important terms relating to coordination compounds, the rules of nomenclature of coordination compounds etc.
By referring to the Class 12 Chemistry Chapter 9 NCERT book solutions, students can understand all the important concepts and practice questions well enough before their examination.
The comprehensive answers given in the CBSE NCERT solutions for Class 12 Chemistry chapter 9 Coordination Compounds will be helpful in understanding the chapter easily.
The revision will be quite easier because the detailed solutions will help you to remember the concepts and get very good marks in your class.
Homework problems won't bother you anymore, all you need to do is check the detailed NCERT Solutions for Class 12 Chemistry and you are good to go.
Also, check NCERT Exemplar Class 12 Solutions
- NCERT Exemplar Class 12th Maths Solutions
- NCERT Exemplar Class 12th Physics Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Class 12th Biology Solutions
Also Check NCERT Books and NCERT Syllabus here:
Frequently Asked Question (FAQs)
Weightage of Coordination Compounds in CBSE Board exam is 6-7 marks. Follow NCERT textbook for a good score in the board exam.
Weightage of Coordination Compounds in JEE mains is 10 marks. To practice questions students can refer to NCERT exemplar and JEE Main previous year papers.
Coordination compounds is a very important chapter for the NEET exam and it holds a weightage of 9% of the total marks.
Also Read
Articles
Certifications By Top Providers
Explore Top Universities Across Globe
Questions related to CBSE Class 12th
Hello aspirant,
The purpose of graphic design extends beyond the brand's look. Nevertheless, by conveying what the brand stands for, it significantly aids in the development of a sense of understanding between a company and its audience. The future in the field of graphic designing is very promising.
There are various courses available for graphic designing. To know more information about these courses and much more details, you can visit our website by clicking on the link given below.
https://www.careers360.com/courses/graphic-designing-course
Thank you
Hope this information helps you.
hello,
Yes you can appear for the compartment paper again since CBSE gives three chances to a candidate to clear his/her exams so you still have two more attempts. However, you can appear for your improvement paper for all subjects but you cannot appear for the ones in which you have failed.
I hope this was helpful!
Good Luck
Hello dear,
If you was not able to clear 1st compartment and now you giving second compartment so YES, you can go for your improvement exam next year but if a student receives an improvement, they are given the opportunity to retake the boards as a private candidate the following year, but there are some requirements. First, the student must pass all of their subjects; if they received a compartment in any subject, they must then pass the compartment exam before being eligible for the improvement.
As you can registered yourself as private candidate for giving your improvement exam of 12 standard CBSE(Central Board of Secondary Education).For that you have to wait for a whole year which is bit difficult for you.
Positive side of waiting for whole year is you have a whole year to preparing yourself for your examination. You have no distraction or something which may causes your failure in the exams. In whole year you have to stay focused on your 12 standard examination for doing well in it. By this you get a highest marks as a comparison of others.
Believe in Yourself! You can make anything happen
All the very best.
Hello Student,
I appreciate your Interest in education. See the improvement is not restricted to one subject or multiple subjects and we cannot say if improvement in one subject in one year leads to improvement in more subjects in coming year.
You just need to have a revision of all subjects what you have completed in the school. have a revision and practice of subjects and concepts helps you better.
All the best.
Hi,
You just need to give the exams for the concerned two subjects in which you have got RT. There is no need to give exam for all of your subjects, you can just fill the form for the two subjects only.
Popular CBSE Class 12th Questions
Colleges After 12th
Also Read
Applications for Admissions are open.

JEE Main Important Physics formulas
ApplyAs per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

UPES School of Liberal Studies
ApplyRanked #52 Among Universities in India by NIRF | Up to 30% Merit-based Scholarships | Lifetime placement assistance | Last Date to Apply - 30th April

Aakash iACST Scholarship Test 2024
ApplyGet up to 90% scholarship on NEET, JEE & Foundation courses

JEE Main Important Chemistry formulas
ApplyAs per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

PACE IIT & Medical, Financial District, Hyd
ApplyEnrol in PACE IIT & Medical, Financial District, Hyd for JEE/NEET preparation

ALLEN JEE Exam Prep
ApplyStart your JEE preparation with ALLEN
Applications for CBSE Class 12 supplementary exams 2023 have started. Read details here.
CBSE Class 12th result 2023 declared. Check now.
