Apply to Aakash iACST Scholarship Test 2024
NCERT Solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
NCERT Solutions for Class 12 Chemistry chapter 10 Haloalkanes and Haloarenes - We all know about the periodic table and its elements. So, ever wondered that from these elements how Haloalkanes and Halaorenes are actually formed? When a hydrogen atom is replaced by a halogen atom (F, Cl, Br, I) in an aliphatic hydrocarbon it results in formation of Haloalkane or alkane halide. The same goes for Haloarenes, when a hydrogen atom is replaced by a halogen in an aromatic hydrocarbon then it forms Haloarene or aryl halide. These haloalkanes and haloarenes ncert solutions will help in understanding concepts of Haloalkanes and Haloarenes. These haloalkanes and haloarenes class 12 chapter 10 are explained by experts in the easiest way. This will help to resolve all NCERT problems of this chapter.
Latest: JEE Main high scoring chapters
Don't Miss: JEE Main 10 year's papers
Recommended: NEET high scoring chapters
Suggested: NEET question papers with solutions
Also Read : Haloalkanes and Haloarenes Class 12 Notes
Topics and Sub-topics of NCERT Solutions for Class 12 Chemistry chapter 10 Haloalkanes and Haloarenes-
Class 12 chemistry ch 10 ncert solutions important topics and subtopics are given below:
10.1 Classification
10.2 Nomenclature
10.3 Nature of C–X Bond
10.4 Methods of Preparation of Haloalkanes
10.5 Preparation of Haloarenes
10.6 Physical Properties
10.7 Chemical Reactions
Find NCERT Solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes below:
Solutions to In-Text Questions Ex 10.1 to 10.9
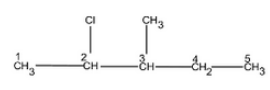
Question 1 Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
Answer :
The structure of 2-Chloro-3-methylpentane is given below :-
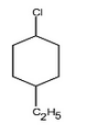
Question 10.1 Write structures of the following compounds:
(ii) 1-Chloro-4-ethylcyclohexane
Answer :
The structure of 1-Chloro-4-ethylcyclohexane is given below :-
Question 10.1 Write structures of the following compounds:
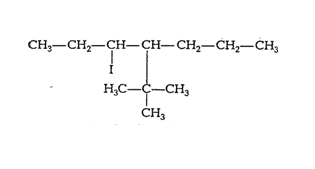
(iii) 4-tert. Butyl-3-iodoheptane
Answer :
The structure of 4-tert. Butyl-3-iodoheptane is given below :-
Question 10.1 Write structures of the following compounds:
Answer :
The structure of 1,4-Dibromobut-2-ene is given below :-

Question 10.1 Write structures of the following compounds:
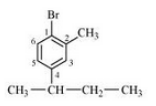
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Answer :
The structure of 1-Bromo-4-sec. butyl-2-methylbenzene is shown below :-
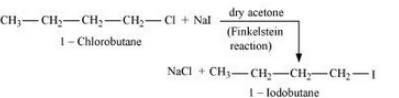
Question 10.2 Why is sulphuric acid not used during the reaction of alcohols with KI?
Answer :
We don't use sulphuric acid because it acts as an oxidising agent and the required alkyl iodide is not produced. The reactions are given below :-
2KI + H 2 SO 4 → 2KHSO 4 + 2HI
2HI + H 2 SO 4 → I 2 + SO 2 + H 2 O
Question 10.3 Write structures of different dihalogen derivatives of propane.
Answer :
We obtain four dihalogen derivatives of propane :-
(i) 1,1 Dibromopropane
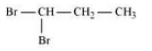
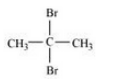
(ii) 2, 2 Dibromopropane
(iii) 1, 2 Dibromopropane
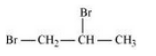
(iv) 1, 3 Dibromopropane
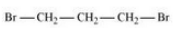
Question 10.4 Among the isomeric alkanes of molecular formula C 5 H 12 , identify the one that on photochemical chlorination yields
Answer :
In this we have to find an isomer in which replacement of any hydrogen atom gives the singel compound for all replacements.
So the isomer is Neopentane.
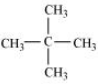
Question 10.4 Among the isomeric alkanes of molecular formula C 5 H 12 , identify the one that on photochemical chlorination yields
(ii) Three isomeric monochlorides.
Answer :
For the given condition we must have three different hydrogens so that we can get three different monochlorides on the replacement.
Thus the isomer is n-pentane.
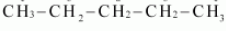
Question 10.4 Among the isomeric alkanes of molecular formula C 5 H 12 , identify the one that on photochemical chlorination yields
(iii) Four isomeric monochlorides .
Answer :
For four monochlorides we need four different hydrogens which can be replaced by chlorine.
Hence the required isomer is :-
Question 10.5 Draw the structures of major monohalo products in each of the following reactions:
(i) 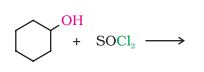
Answer:
The final products are:-
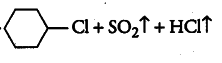
Question 10.5 Draw the structures of major monohalo products in each of the following reactions:
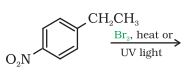
Answer :
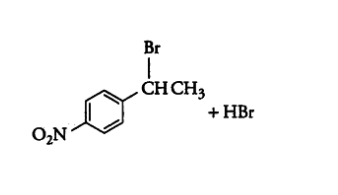
Question 10.5 Draw the structures of major monohalo products in each of the following reactions:
(iii) 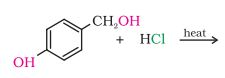
Answer :
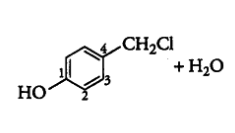
Question 10.5 Draw the structures of major monohalo products in each of the following reactions:
(iv) 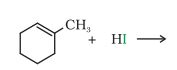
Answer :
The obtained product is:-
Question 10.5 (V) Draw the structures of major monohalo products in each of the following reactions:
Answer : 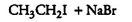
Question 10.5 Draw the structures of major monohalo products in each of the following reactions:
(vi) 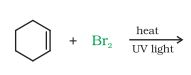
Answer :
The obtained product is :-
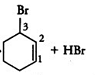
Question 10.6 Arrange each set of compounds in order of increasing boiling points.
(i) Bromomethane, Bromoform, Chloromethane, Dibromomethane.
Answer :
It is known that boiling point increases with increase in molecular mass when the alkyl group is the same.
So the order of increasing boiling point is Chloromethane < Bromomethane < Dibromomethane < Bromoform
Question 10.6 Arrange each set of compounds in order of increasing boiling points.
(ii) 1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
Answer :
In the given compounds the halide groups are same. In these cases, the boiling point depends on the bulkiness of the alkyl group. The boiling point increases with an increase in the chain length. Also, the boiling point decreases with an increase in branching.
So the order is :- 1- Chlorobutane > 1- Chloropropane > Isopropyl Chloride
Question 10.7 Which alkyl halide from the following pairs would you expect to react more rapidly by an mechanism? Explain your answer.
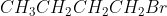
Answer :
In this case, the rate of reaction will depend on the hindrance of the substrate.
Since 1- Bromobutane is a alkyl halide and 2- Bromobutane is a
alkyl halide hence 2- Bromobutane gives more hindrance to the nucleophile.
Hence 1- Bromobutane reacts faster.
Question 10.7 Which alkyl halide from the following pairs would you expect to react more rapidly by an mechanism? Explain your answer.

Answer :
The rate of reaction decreases with increase in hindrance to the attack of the nucleophile.
So 2-bromobutane will react faster than 2-bromo-2-methylpropane in the nucleophilic attack.
Question 10.7 Which alkyl halide from the following pairs would you expect to react more rapidly by an mechanism? Explain your answer.
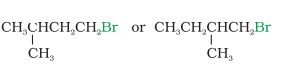
Answer :
In these kinds of cases, we see where is the substituent is attached i.e., how far from the halide group. It can be clearly seen that the methyl group attached in 1-bromo-2-methylbutane is near than that attached in 1-bromo-3-methyl butane.
Hence the rate of reaction will be faster in case of 1-bromo-3-methylbutane.
Question 10.8 In the following pairs of halogen compounds, which compound undergoes faster reaction?
(i) 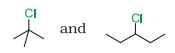
Answer :
In reactions, we see the formation of carbocation and this is the rate determining step for this kind of reactions. So the compound having more stable carbocation will react faster. In the given case 2- Chloro, 2- Methylpropane we have
carbon whereas in 3- Chloropentane we have
carbon.
Question 10.8 In the following pairs of halogen compounds, which compound undergoes faster reaction?
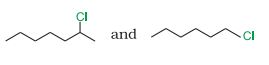
Answer :
In reactions, we see the formation of carbocation and this is the rate determining step for these kinds of reactions.So the compound having more stable carbocation will react faster. Hence 2-Chloroheptane will react faster than 1- Chlorohexane.
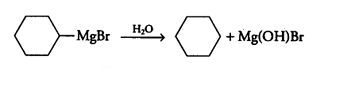
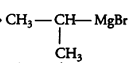
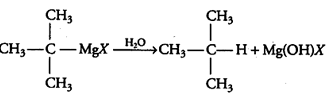
Question 10.9 Identify A, B, C, D, E, R and R1 in the following:
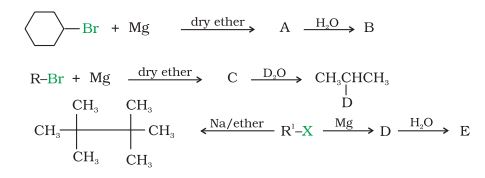
Answer :
1st reaction :-
2nd reaction :-
3rd reaction :-
NCERT Solutions for Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes- Exercise Questions
(i)
Answer :
(i) 2-Chloro-3-methylbutane. And it is a secondary alkyl halide.
(ii)
Answer :
3-Chloro-4-methylhexane. And it is primary alkyl halide.
(iii)
Answer :
(iii) 1-Iodo-2, 2-dimethylbutane. And it is primary alkyl halide.
Question 10.1 Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
(iv)
Answer :
(iv) 1-Bromo-3, 3-dimethyl-1-phenylbutane. And it is secondary benzyl halide.
(v)
Answer :
(v) 2-Bromo-3-methylbutane. And it is secondary alkyl halide.
(vi)
Answer :
(vi) 1-Bromo-2-ethyl-2-methylbutane. And it is a primary alkyl halide.
Question 10.1 Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
(vii)
Answer :
(vii) 3-Chloro-3-methylpentane. And it is tertiary alkyl halide.
(viii)
Answer :
(viii) 3-Chloro-5-methylhex-2-ene. And it is vinyl halide
(ix)
Answer :
(ix) 4-Bromo-4-methylpent-2-ene. And it is allyl halide.
(x)
Answer :
(x) 1-Chloro-4-(2-methylpropyl) benzene. And it is aryl halide.
Question 10.1 Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
(xi)
Answer :
(xi) 1-Chloromethyl-3-(2, 2-dimethylpropyl) benzene. And it is primary benzyl halide.
(xii)
Answer :
(xii) 1-Bromo-2-(1-methylpropyl) benzene. And it is aryl halide.
Question 10.2 Give the IUPAC names of the following compounds:
(i)
Answer :
(i) 2-Bromo-3-chlorobutane
Question 10.2 Give the IUPAC names of the following compounds:
(ii)
Answer :
1-Bromo-1-chloro-1, 2, 2-trifluroethane
Question 10.2 Give the IUPAC names of the following compounds:
(iii)
Answer :
1-Bromo-4-chlorobut-2-yne
Question 10.2 Give the IUPAC names of the following compounds:
(iv)
Answer :
2-(Trichloromethyl)-1, 1, 1, 2, 3, 3, 3-heptachloropropane
Question 10.2 Give the IUPAC names of the following compounds:
(v)
Answer :
2-Bromo-3, 3-bis(4-Chlorophenyl) butane
Question 10.2 Give the IUPAC names of the following compounds:
(vi)
Answer :
1-Chloro-1-(4-iodophenyl)-3, 3-dimethylbut-1-ene
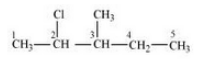
Question 10.3 Write the structures of the following organic halogen compounds.
(i) 2-Chloro-3-methylpentane
Answer :
(i)
Question 10.3 Write the structures of the following organic halogen compounds.
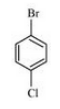
(ii) p-Bromochlorobenzene
Answer :
(ii)
Question 10.3 Write the structures of the following organic halogen compounds.
(iii) 1-Chloro-4-ethylcyclohexane
Answer :
(iii)

Question 10.3 Write the structures of the following organic halogen compounds.
(iv) 2-(2-Chlorophenyl)-1-iodooctane
Answer :
(iv)
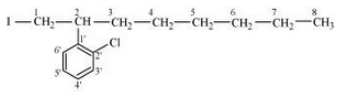
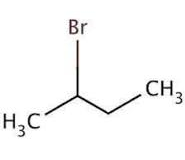
Question 10.3 Write the structures of the following organic halogen compounds.
(v) 2-Bromobutane
Answer :
(v)
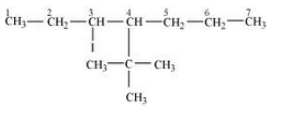
Question 10.3 Write the structures of the following organic halogen compounds.
(vi) 4-tert-Butyl-3-iodoheptane
Answer :
(vi)
Question 10.3 Write the structures of the following organic halogen compounds.
(vii) 1-Bromo-4-sec-butyl-2-methylbenzene
Answer :
(vii)
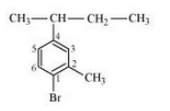
Question 10.3 Write the structures of the following organic halogen compounds.
(viii) 1,4-Dibromobut-2-ene
Answer :
(viii)
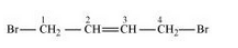
Question 10.4 Which one of the following has the highest dipole moment?
(i) CH 2 Cl 2 (ii) CHCl 3 (iii) CCl 4
Answer :
The order of dipole moment will be :- CH 2 Cl 2 > CHCl 3 > CCl 4.
The reason for the above order is given as- CCl 4 is a symmetrical compound so its dipole moment will be zero. In case of CHCl 3 , one of the Cl cancels dipole moment of the opposite Cl atom, so net dipole moment is just due to one Cl. In the case of CH 2 Cl 2 , both Cl groups contribute to the dipole moment so it has the highest dipole moment among all.
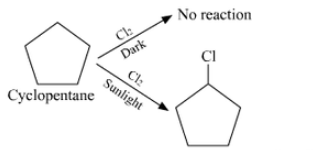
Answer :
We are given the formula C 5 H 10 which can be either of an alkene or of cycloalkane. Since the hydrocarbon doesn't react with chlorine in dark thus it cannot be alkene. So the only option left out is cyclopentane.
Question 10.6 Write the isomers of the compound having formula .
Answer :
The isomers of the compound are :-
(i) 1-Bromobutane
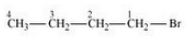
(ii) 2-Bromobutane
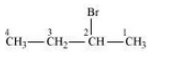
(iii) 1-Bromo-2-methylpropane
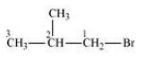
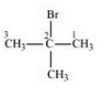
(iv) 2-Bromo-2-methylpropane
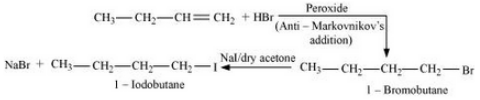
Question 10.7 Write the equations for the preparation of 1-iodobutane from:
(i) 1-butanol
Answer :
(i) The procedure given below can be used :-

Question 10.7 Write the equations for the preparation of 1-iodobutane from
Answer :
(iii) The required product is obtained by following procedure :-
Question 10.8 What are ambident nucleophiles? Explain with an example.
Answer :
The ambident nucleophiles are those nucleophiles which have two nucleophilic sites through which they can attack. For e.g Nitrile ion can attack through both nitrogen atom (forms nitroalkanes) and an oxygen atom (forms alkyl nitrites), thus it is an ambident nucleophile.
Question 10.9 Which compound in each of the following pairs will react faster in S N 2 reaction with –OH?
Answer :
In this case, we have the same alkyl group but different halide ions. For this rate of S N 2 reaction increases with increase in atomic mass. So, CH 3 I will react faster than CH 3 Br.
Question 10.9 Which compound in each of the following pairs will react faster in S N 2 reaction with –OH?
Answer :
In this case, the hindrance will be deciding factor for the rate of S N 2 reaction because hindrance will directly affect the attack of the nucleophile. So will react faster as compared to
.
Question 10.10 Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
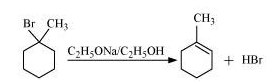
(i) 1-Bromo-1-methylcyclohexane
Answer :
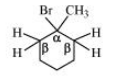
In this compound, it is clear that we have identical hydrogen, therefore, dehalogenation of the given compound gives the same alkene.
Question 10.10 Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
Answer :
(ii)
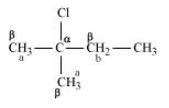
In this compound we have two kind of hydrogen. So dehalogenation will give two kind of alkenes, namely 2-Methylbut-2-ene and 2-Methylbut-1-ene.
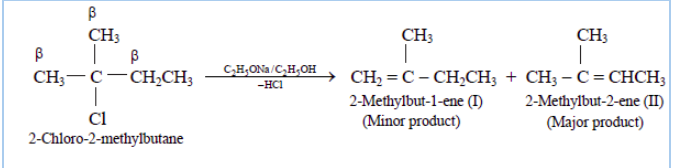
![]()
The major product of this reaction will be 2-Methylbut-2-ene as the number of - hydrogens attached to double bonded carbon are more in case of this compound.
Question 10.10 Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(iii) 2,2,3-Trimethyl-3-bromopentane
Answer :
(iii)
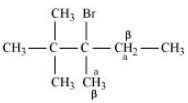
In this compound we have two type of - hydrogen thus dehalogenation we get two types of products namely 3, 4, 4-Trimethylpent-2-ene and 2-Ethyl-3,3-dimethylbut-2-ene.
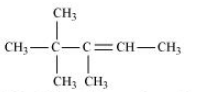 and
and 
Here 3, 4, 4-Trimethylpent-2-ene will be major product, since the - hydrogen attached to the double bond are greater.
Question 10.11 How will you bring about the following conversions?
Answer :
(i) The conversion will take place by following procedure :-

Now,
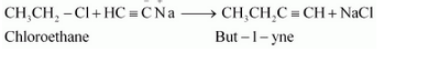
Question 10.11 How will you bring about the following conversions?
Answer :
(ii)
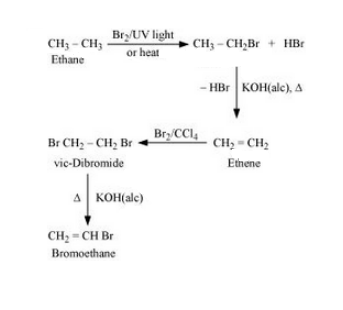
Question 10.11 How will you bring about the following conversions?
(iii) Propene to 1-nitropropane
Answer :
(iii)
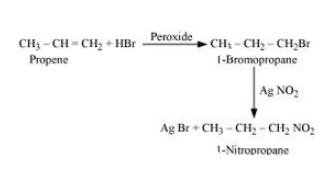
Question 10.11 How will you bring about the following conversions?
(iv) Toluene to benzyl alcohol
Answer :
(iv)
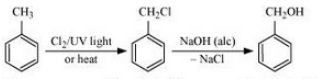
Question 10.11 How will you bring about the following conversions?
Answer :
(v)
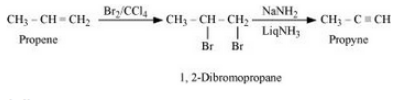
Question 10.11 How will you bring about the following conversions?
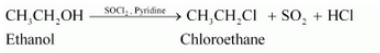
(vi) Ethanol to ethyl fluoride
Answer :
(vi)
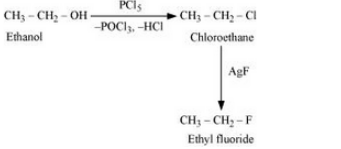
Question 10.11 How will you bring about the following conversions?
(vii) Bromomethane to propanone
Answer :
(vii)
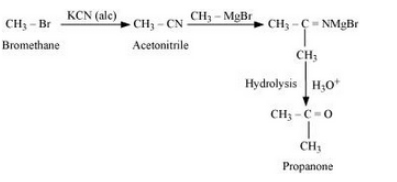
Question 10.11 How will you bring about the following conversions?
Answer :
(viii)
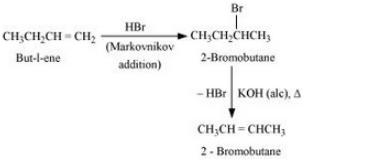
Question 10.11 How will you bring about the following conversions?
(ix) 1-Chlorobutane to n-octane
Answer :
(ix)
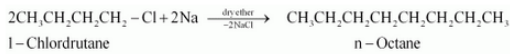
Question 10.11 How will you bring about the following conversions?
Answer :
(x)
Question 10.12 Explain why
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
Answer :
We know that the Cl-atom in chlorobenzene is attached to a sp 2 hybridized carbon atom whereas, in cyclohexyl chloride, the Cl-atom is attached to a sp 3 hybridized carbon atom. It is known that sp 2 hybridized carbon has more s-character than sp 3 hybridized carbon atom. Thus, chlorobenzene is more electronegative than cyclohexyl chloride.
Apart from this, the - R effect of the benzene ring of chlorobenzene results in decreasing the electron density of the C - Cl bond near the Cl-atom. And the C - Cl bond in chlorobenzene becomes less polar. Due to these reasons, the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Question 10.12 Explain why
(ii) alkyl halides, though polar, are immiscible with water?
Answer :
For being soluble in the water we have a condition that the solute-water force of attraction must be stronger than the solute-solute and water-water forces of attraction. Alkyl halides are held together by dipole-dipole interactions and there are polar molecules. Similarly, the intermolecular force of attraction present between the water molecules is hydrogen bonding. The new force of attraction after we dissolve solute in water i.e., between the alkyl halides and water molecules is weaker than the alkyl halide-alkyl halide and water-water forces of attraction. That is why alkyl halides (though polar) are immiscible with water.
Question 10.12 Explain why
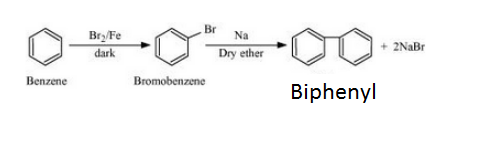
(iii) Grignard reagents should be prepared under anhydrous conditions?
Answer :
This is done because in presence of moisture, they react to give alkane.
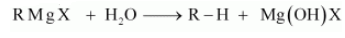
Question 10.13 Give the uses of freon 12, DDT, carbon tetrachloride and iodoform.
Answer :
(i) Freon-12 or dichlorodifluoromethane is generally known as CFC. It is used in refrigerators and air conditioners as a refrigerant. It is also used in body sprays, hair sprays, etc. But it has environmental impacts as it damages the ozone layer.
(ii) DDT or p, p-dichlorodiphenyltrichloroethane is one of the best-known insecticides which was used very widely all over the world. It is very effective against mosquitoes, insects and lice. But it has harmful effects.
(iii) CCl 4 :- It is mostly used for manufacturing refrigerants for refrigerators and air conditioners. It is also used as a solvent in the manufacture of pharmaceutical products. In the early years, carbon tetrachloride was widely used as a cleaning fluid and a fire extinguisher.
(iv) Iodoform was used earlier as an antiseptic. And this antiseptic property of iodoform is due to the liberation of free iodine when it comes in contact with the skin.
Question 10.14 Write the structure of the major organic product in each of the following reactions:
(i)
Answer :
(i) The obtained product is :-
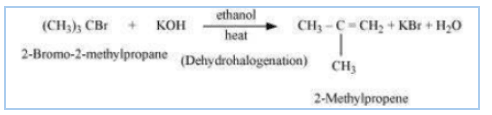
Question 10.14 Write the structure of the major organic product in each of the following reactions:
(ii)
Answer :
(ii) The obtained product is 2-Methylpropene
![]()
Question 10.14 Write the structure of the major organic product in each of the following reactions:
(iii)
Answer :
(iii) The obtained product is Butan-2-ol.
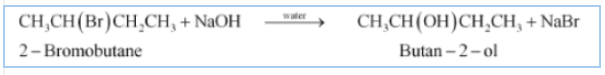
![]()
Question 10.14 Write the structure of the major organic product in each of the following reactions:
(iv)
Answer :
(iv) The obtained product is Cyanoethane.

Question 10.14 Write the structure of the major organic product in each of the following reactions:
(v)
Answer :
(v) The obtained product is Phenetole.
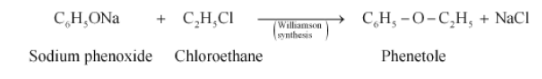
Question 10.14 Write the structure of the major organic product in each of the following reactions:
(vi)
Answer :
(vi) The obtained product is 1-Chloropropane.
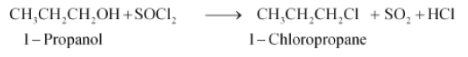
Question 10.14 Write the structure of the major organic product in each of the following reactions:
(vii)
Answer :
(vii) The obtained product is 1-Bromobutane.

Question 10.14 Write the structure of the major organic product in each of the following reactions:
(viii)
Answer :
The obtained product is 2-Bromo-2-methylbutane.
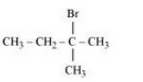
Question 10.15 Write the mechanism of the following reaction:
Answer :
The reaction will proceed through S N 2 mechanism. The mechanism for the given reaction is shown below :-
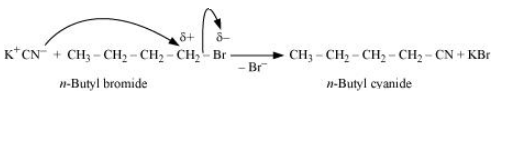
Question 10.16 Arrange the compounds of each set in order of reactivity towards S N 2 displacement:
(i) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
Answer :
(i) Here the deciding factor the rate of reaction will be a steric hindrance.
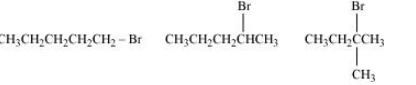
It is clear from the above that the order of hindrance is:-
1-Bromopentane < 2-bromopentane < 2-Bromo-2-methylbutane
So the order of rate of reaction will be:-
2-Bromo-2-methylbutane < 2-Bromopentane < 1-Bromopentane
Question 10.16 Arrange the compounds of each set in order of reactivity towards S N 2 displacement:
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
Answer :
(ii) In this case, also, the order of the rate of reaction will be decided from the steric hindrance factor.
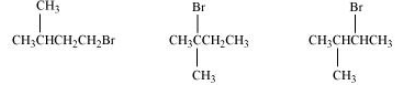
It is clear from the above that the steric hindrance in 2-Bromo-2-methylbutane highest (note that hindrance of the carbon attached to halide ion is seen). So the order of the rate of reaction is:-
2-Bromo-2-methylbutane < 2-Bromo-3-methylbutane < 1-Bromo-3-methylbutane
Question 10.16 Arrange the compounds of each set in order of reactivity towards S N 2 displacement:
(iii) 1-Bromobutane, 1-Bromo-2,2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane.
Answer :
(iii) The steric hindrance is the deciding factor here.
The order of steric hindrance is :-
1-Bromobutane < 1-Bromo-3-methylbutane < 1-Bromo-2-methylbutane< 1-Bromo-2, 2-dimethylpropane
Thus the order of the rate of reaction will be : -
1-Bromo-2, 2-dimethylpropane < 1-Bromo-2-methylbutane < 1-Bromo-3- methylbutane < 1-Bromobutane
Question 10.17 Out of and
, which is more easily hydrolysed by aqueous KOH.
Answer :
Hydrolysis by KOH will take place by the formation of a carbocation in its rate-determining step. So the compound having stable carbocation will hydrolyse faster.
The carbocations of both the compounds are given below:-
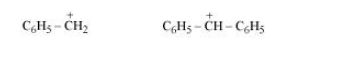
It is clear that carbocation of is more stable.
Hence will hydrolyse faster than
.
Question 10.18 p-Dichlorobenzene has higher m.p. than those of o- and m-isomers. Discuss.
Answer :
The structures of o-Dichlorobenzene, m-Dichlorobenzene and p-Dichlorobenzene are given below.
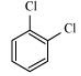
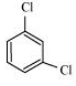
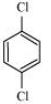
We can see that p-Dichlorobenzene is a very symmetric structure thus packing of it will be maximum. As a result more and more energy will be required to break bonds (during boiling). Thus boiling point is high for p-Dichlorobenzene.
Question 10.19 How the following conversions can be carried out?
Answer :
The mechanism is given below :-
Question 10.19 How the following conversions can be carried out?
Answer :
The reaction mechanism is given below :-
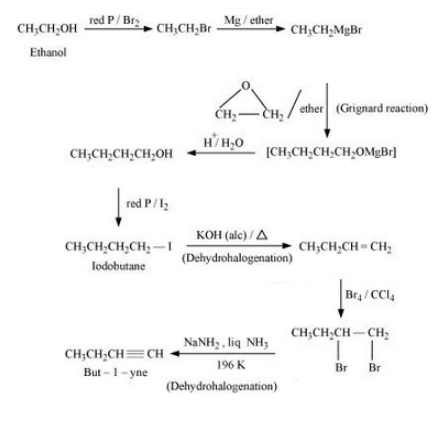
Question 10.19 How the following conversions can be carried out?
(iii) 1-Bromopropane to 2-bromopropane
Answer :
(iii) The mechanism is given below :-
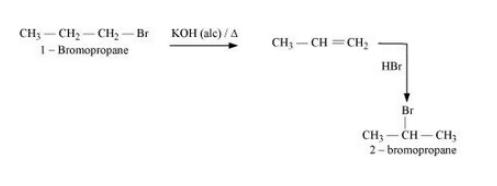
Question 10.19 How the following conversions can be carried out?
(iv) Toluene to benzyl alcohol
Answer :
(iv) The mechanism is given below :-
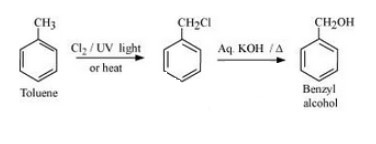
Question 10.19 How the following conversions can be carried out?
(v) Benzene to 4-bromonitrobenzene
Answer :
The mechanism for the given reaction is as follows :-
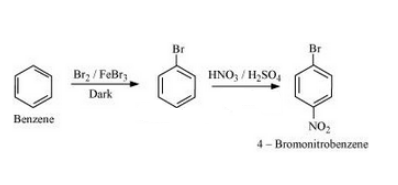
Question 10.19 How the following conversions can be carried out?
(vi) Benzyl alcohol to 2-phenylethanoic acid
Answer :
(vi) The mechanism for the reaction is given below :-
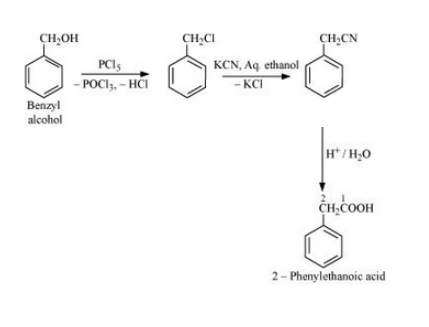
Question 10.19 How the following conversions can be carried out?
(vii) Ethanol to propanenitrile
Answer :
(vii) The mechanism of the given reaction reaction is :-
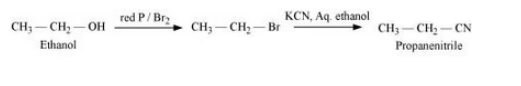
Question 10.19 How the following conversions can be carried out?
(viii) Aniline to chlorobenzene
Answer :
The mechanism of the reaction is given below :-
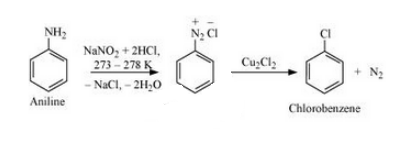
Question 10.19 How the following conversions can be carried out ?
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
Answer :
(ix) The required mechanism is as follows :-
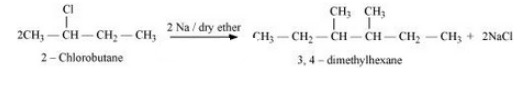
Question 10.19 How the following conversions can be carried out?
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
Answer :
(x) The required mechanism is given below :-
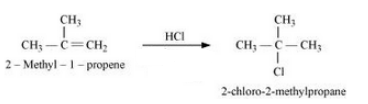
Question 10.19 How the following conversions can be carried out ?
(xi) Ethyl chloride to propanoic acid
Answer :
The mechanism of the given reaction is :-
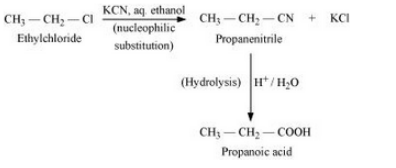
Question 10.19 How the following conversions can be carried out?
(xii) But-1-ene to n-butyliodide
Answer :
The mechanism is given below :-
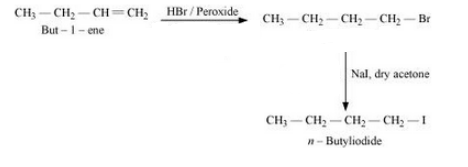
Question 10.19 How the following conversions can be carried out?
(xiii) 2-Chloropropane to 1-propanol
Answer :
The mechanism is :-
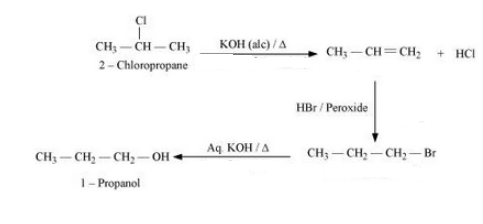
Question 10.19 How the following conversions can be carried out?
(xiv) Isopropyl alcohol to iodoform
Answer :
(xiv) The proposed mechanism is :-
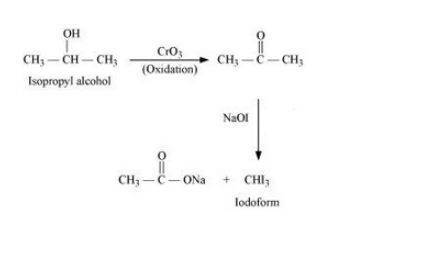
Question 10.19 How the following conversions can be carried out?
(xv) Chlorobenzene to p-nitrophenol
Answer :
(xv) The required mechanism is given below :-
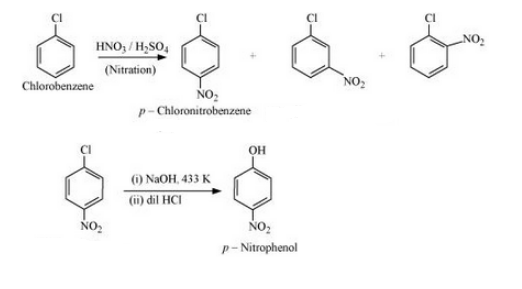 `
`
Question 10.19 How the following conversions can be carried out?
(xvi) 2-Bromopropane to 1-bromopropane
Answer :
The mechanism of the reaction is given below :-
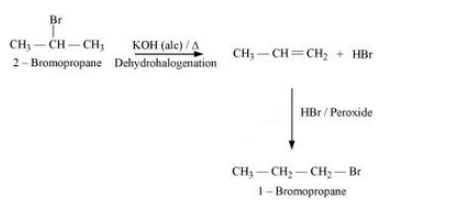
Question 10.19 How the following conversions can be carried out?
Answer :
(xvii) The mechanism of the reaction is :-
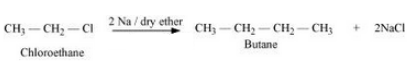
Question 10.19 How the following conversions can be carried out?
Answer :
The mechanism for the given reaction is :-

Question 10.19 How the following conversions can be carried out?
(xix) tert-Butyl bromide to isobutyl bromide
Answer :
The mechanism of the given reaction is :-
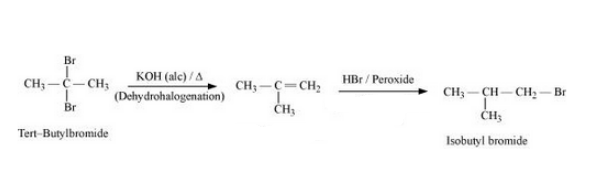
Question 10.19 How the following conversions can be carried out?
(xx) Aniline to phenylisocyanide
Answer :
The mechanism for the given reaction is as follows :-
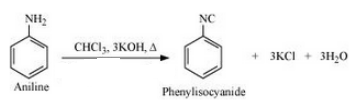
Answer :
In an aqueous solution, KOH almost completely dissociates into OH - ions. We know that OH - ions are strong nucleophile, which leads the alkyl chloride to undergo a reaction to form alcohol. But an alcoholic solution of KOH contains alkoxide (RO - ) ion, which is a strong base. Thus, it can remove hydrogen from the β-carbon of the alkyl chloride and form an alkene. The OH - ion is a weaker base than RO - ion. The basic character of OH - ion decreases in aqueous solution. Therefore, it cannot remove hydrogen from the β-carbon.

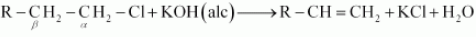
Answer :
With the given formula we have two alkyl halides They are n - butyl bromide and isobutyl bromide.
For the first set of reaction we get two possibilities:-
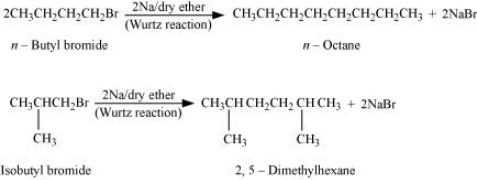
Therefore compound (d) is 2, 5-dimethylhexane.

Question 10.22 What happens when
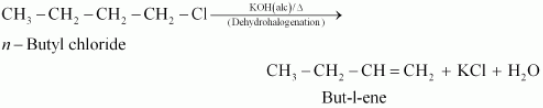
(i) n-butyl chloride is treated with alcoholic KOH,
Answer :
When n-butyl chloride is treated with alcoholic KOH the following reaction occurs:-
Question 10.22 What happens when
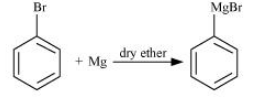
(ii) bromobenzene is treated with Mg in the presence of dry ether
Answer :
When bromobenzene is treated with Mg in the presence of dry ether the following reaction occurs :-
Question 10.22 What happens when
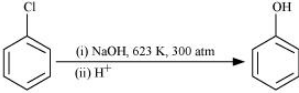
(iii) chlorobenzene is subjected to hydrolysis
Answer :
The reaction is given below :-
Question 10.22 What happens when
(iv) ethyl chloride is treated with aqueous KOH
Answer :
When ethyl chloride is treated with aqueous KOH the following reaction occurs :-

Question 10.22 What happens when
(v) methyl bromide is treated with sodium in the presence of dry ether
Answer :
When methyl bromide is treated with sodium in the presence of dry ether then following reaction occurs:-

Question 10.22 What happens when
(vi) methyl chloride is treated with KCN?
Answer :
When methyl chloride is treated with KCN the following reaction occurs:-

More About NCERT Solutions for Class 12 Chemistry Chapter 10 Haloalkanes And Haloarenes
Our body produces thyroxine hormone which contains iodine and deficiency of iodine can cause a disease called goiter. In this chapter, you will study methods of preparation, physical and chemical properties and uses of haloalkanes and haloarenes. You will find all the NCERT solutions for class 12 chemistry chapter 10 Haloalkanes and Haloarenes here for free.
Also Read| NCERT Exemplar Solutions For Haloalkanes And Haloarenes
This NCERT syllabus chapter carries 4 marks in the CBSE board exams hence it becomes necessary for you to find all the Class 12 Chemistry chapter 10 NCERT solutions haloalkanes and haloarenes to score good marks. In this NCERT book chapter, there are 9 intext questions and 22 questions in the exercise and you will get complete NCERT Solutions for Class 12 for reference.
These NCERT Solutions will help you in your preparation for CBSE Board exams as well as in competitive exams like JEE, NEET, BITSAT, and KVPY, etc. The seven sub-topics of NCERT solutions for Class 12 Chemistry chapter 10 Haloalkanes and Haloarenes covers important concepts like IUPAC nomenclature and preparation of haloalkanes and haloarenes and discuss how to use stereochemistry as a tool in the understanding the reaction mechanism and applications of organo-metallic compounds.
At the end of the chapter, the environmental effects of polyhalogen compounds are also highlighted. Halogenated compounds remain in the environment due to their resistance to breakdown by the bacteria in the soil. By referring to the NCERT Class 12 Chemistry solutions chapter 10, students can understand all the important concepts and practice questions well enough before their examination. Read the entire article to know more on Class 12 Chemistry chapter 10 NCERT solutions.
Some Important Basic Points of the NCERT Class 12 Chemistry Solutions Chapter 10
The replacement of one or more than one H-atom in an aliphatic hydrocarbon by halogen atoms results in the formation haloalkane or alkyl halide and the replacement of H-atoms in an aromatic hydrocarbon by halogen atoms results in the formation of haloarene or aryl halide.
In haloalkanes, halogen atoms attached to the
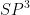 hybridised carbon atom of an alkyl group.
hybridised carbon atom of an alkyl group.in haloarenes, halogen atoms attached to the
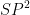 hybridised carbon atoms of an aryl group.
hybridised carbon atoms of an aryl group.For better understanding use NCERT Solutions for Class 12 Chemistry chapter 10 PDF, students can download it by using an online webpage to PDF converter
NCERT Solutions Class 12 Chemistry
NCERT Solutions for Class 12 Subject wise
- NCERT Solutions for class 12 biology
- NCERT solutions for class 12 maths
- NCERT solutions for class 12 chemistry
- NCERT Solutions for class 12 physics
SAT® | CollegeBoard
Registeration closing on 19th Apr for SAT® | One Test-Many Universities | 90% discount on registrations fee | Free Practice | Multiple Attempts | no penalty for guessing
TOEFL ® Registrations 2024
Thinking of Studying Abroad? Think the TOEFL® test. Save 10% on your TOEFL exam with ApplyShop gift cards!
Benefits of NCERT solutions for class 12 chemistry chapter 10 Haloalkanes and Haloarenes
The detailed and comprehensive answers given in the NCERT Class 12 Chemistry solutions chapter 10 will be helpful in understanding the chapter easily.
Revision will be quite easier because the detailed solutions will help you to remember the concepts and get good marks in your class.
Homework problems won't bother you anymore, all you need to do is check the detailed NCERT solutions for Class 12 Chemistry and you are good to go.
If you have a doubt or question, these Class 12 Chemistry chapter 10 NCERT solutions will help you quickly. You will get all the answers that will help you score well in your exams.
Also Check NCERT Books and NCERT Syllabus here:
Frequently Asked Question (FAQs)
Below is the list of important topics:
Preparation of haloalkanes
Physical properties of halo derivatives
Reactions of Haloalkanes Stereoisomerism Definition: Enantiomers, Racemic mixture, Racemisation Reactions of haloarenes
Weightage of NCERT class 12 Chemistry chapter 10 in CBSE board exam is 4 marks. Follow NCERT syllabus and NCERT textbook for a good score in the exam. To practice more questions NCERT exemplar can also be used.
Also Read
Articles
Certifications By Top Providers
Explore Top Universities Across Globe
Questions related to CBSE Class 12th
Hello aspirant,
The purpose of graphic design extends beyond the brand's look. Nevertheless, by conveying what the brand stands for, it significantly aids in the development of a sense of understanding between a company and its audience. The future in the field of graphic designing is very promising.
There are various courses available for graphic designing. To know more information about these courses and much more details, you can visit our website by clicking on the link given below.
https://www.careers360.com/courses/graphic-designing-course
Thank you
Hope this information helps you.
hello,
Yes you can appear for the compartment paper again since CBSE gives three chances to a candidate to clear his/her exams so you still have two more attempts. However, you can appear for your improvement paper for all subjects but you cannot appear for the ones in which you have failed.
I hope this was helpful!
Good Luck
Hello dear,
If you was not able to clear 1st compartment and now you giving second compartment so YES, you can go for your improvement exam next year but if a student receives an improvement, they are given the opportunity to retake the boards as a private candidate the following year, but there are some requirements. First, the student must pass all of their subjects; if they received a compartment in any subject, they must then pass the compartment exam before being eligible for the improvement.
As you can registered yourself as private candidate for giving your improvement exam of 12 standard CBSE(Central Board of Secondary Education).For that you have to wait for a whole year which is bit difficult for you.
Positive side of waiting for whole year is you have a whole year to preparing yourself for your examination. You have no distraction or something which may causes your failure in the exams. In whole year you have to stay focused on your 12 standard examination for doing well in it. By this you get a highest marks as a comparison of others.
Believe in Yourself! You can make anything happen
All the very best.
Hello Student,
I appreciate your Interest in education. See the improvement is not restricted to one subject or multiple subjects and we cannot say if improvement in one subject in one year leads to improvement in more subjects in coming year.
You just need to have a revision of all subjects what you have completed in the school. have a revision and practice of subjects and concepts helps you better.
All the best.
Hi,
You just need to give the exams for the concerned two subjects in which you have got RT. There is no need to give exam for all of your subjects, you can just fill the form for the two subjects only.
Popular CBSE Class 12th Questions
Colleges After 12th
Also Read
Applications for Admissions are open.

JEE Main Important Physics formulas
ApplyAs per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

UPES School of Liberal Studies
ApplyRanked #52 Among Universities in India by NIRF | Up to 30% Merit-based Scholarships | Lifetime placement assistance

Aakash iACST Scholarship Test 2024
ApplyGet up to 90% scholarship on NEET, JEE & Foundation courses

JEE Main Important Chemistry formulas
ApplyAs per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

PACE IIT & Medical, Financial District, Hyd
ApplyEnrol in PACE IIT & Medical, Financial District, Hyd for JEE/NEET preparation

ALLEN JEE Exam Prep
ApplyStart your JEE preparation with ALLEN