Apply to Aakash iACST Scholarship Test 2024
NCERT Solutions for Class 12 Chemistry Chapter 13 Amines
NCERT Solutions for Class 12 Chemistry Chapter 13 Amines - You will find amines ncert solutions class 12 one of the important organic molecules which are present in proteins, vitamins, and hormones, etc. In Amines Class 12 chapter, you will deal with organic compounds of amines and learn about amines and diazonium salts. You may also have some doubts which can be resolved by going through NCERT solutions for Class 12 Chemistry chapter 13 Amines. Amines are also considered as derivatives of ammonia.
Latest: JEE Main high scoring chapters
Don't Miss: JEE Main 10 year's papers
Recommended: NEET high scoring chapters
Suggested: NEET question papers with solutions
Also Read
In Amines Class 12 NCERT chapter, there are 14 questions in the exercise. The NCERT solutions for class 12 chemistry chapter 13 Amines are prepared in a very detailed manner which has all the answers to questions. Chemistry chapter 13 Amines holds four marks in the class 12 CBSE board exam. These amines class 12 ncert solutions help students in their preparation of Cclass 12 CBSE Board exam as well as various competitive exams like JEE Mains, NEET, etc. Scroll down to get NCERT solutions for Amines Class 12. By referring to the NCERT solutions for class 12 , students can understand all the important concepts and practice questions well enough before their examination.
NCERT Solutions for Class 12 Chemistry Chapter 13 Amines- Exercise Questions
Solutions to In-Text Questions Ex 13.1 to 13.9
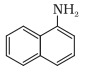
Question 13.1 Classify the following amines as primary, secondary or tertiary:
Answer :
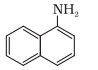
N atom is directly connected with only one C atom, so it is a primary aromatic amine
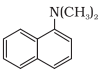
Question 13.1 Classify the following amines as primary, secondary or tertiary:
Answer :
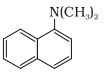
In this compound N atom directly connected with 3 carbon atoms. So, it is a tertiary aromatic amine
Question 13.1 Classify the following amines as primary, secondary or tertiary:
Answer :
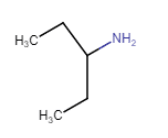
Here N atom directly connected with only one C atom. So it is a primary aliphatic amine.
Question 13.1 Classify the following amines as primary, secondary or tertiary:
Answer :
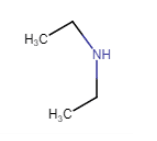
In structure, we can clearly see that N is directly connected with 2 carbon atom. so, it is a secondary amine.
Question 13.2 (i) Write structures of different isomeric amines corresponding to the molecular formula,
Answer :
different isomeric amines corresponding to the molecular formula,
(i ) (v)
(ii) 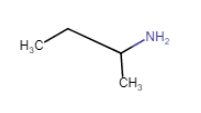 (vi)
(vi) 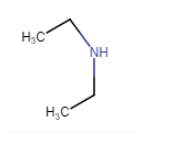
(iii) 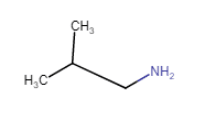
![]() (vii)
(vii) 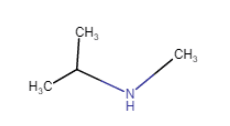
(iv) 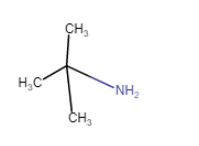 (viii)
(viii) 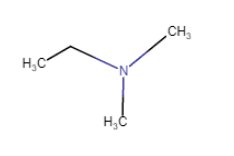
Question 13.2 (ii) Write IUPAC names of all the isomers.
Answer :
IUPAC name of all the isomers-
Butanamine | |
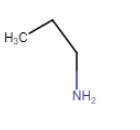 | Butan-2-amine |
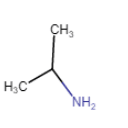 | 2-Methylpropanamine |
 | N-Methylpropan-2-amine |
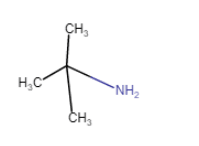 | 2-Methylpropan-2-amine |
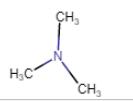
 | N,N-Dimethylethanamine |
N-Methylpropanamine | |
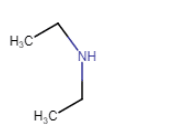 | N-Ethylethanamine |
Question 13.2(iii) What type of isomerism is exhibited by different pairs of amines?
Answer :
Butanamine (Chain isomerism + position isomerism) | |
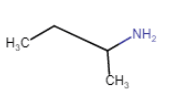 | Butan-2-amine (chain isomerism + position isomerism) |
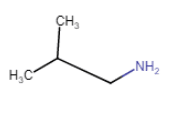 | 2-Methylpropanamine (chain isomerism) |
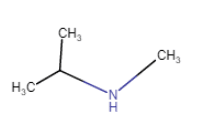 | N-Methylpropan-2-amine (position isomerism + metamerism) |
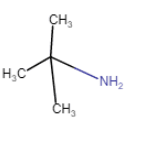 | 2-Methylpropan-2-amine (chain isomerism) |
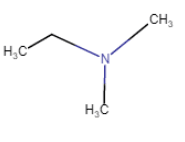 | N,N-Dimethylethanamine |
N-Methylpropanamine (position isomerism) | |
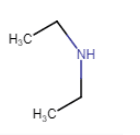 | N-Ethylethanamine (no isomerism) |
Question 13.3(i) How will you convert
Answer :
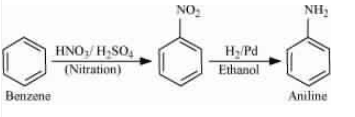
Nitration of benzene gives nitrobenzene. And now reduce the nitro group by catalytic hydrogenation.
Question 13.3(ii) How will you convert
Benzene into N, N-dimethylaniline
Answer :
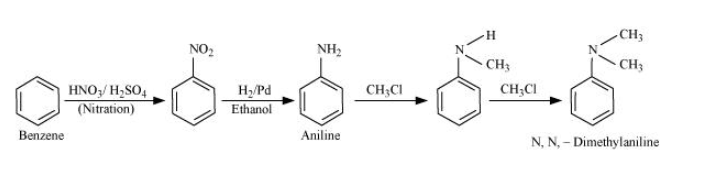
Nitration of benzene gives nitrobenzene and after catalytic hydrogenation of nitrobenzene, it gives aniline. Aniline on reacting with two moles of chloromethane to form N, N-dimethylaniline.
Question 13.3(iii) How will you convert
Answer :
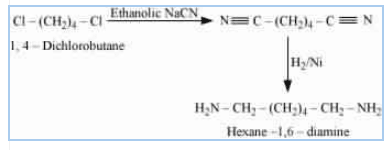
ON reacting the given reactant with ethanolic sodium cyanide, the CN molecules replace both chlorine atom. And after that catalytic hydrogenation, we get our desired product. (reduce the CN to in both sides)
Question 13.4(i) Arrange the following in increasing order of their basic strength:
Answer :
Considering the inductive effect, solvation effect and steric hindrance of the alkyl group which decides the basic strength of alkylamines. order of basic strength in ethyl substituted amine is-
and order in benzene substituted ring-
Due to the -R effect of benzene has less electron density than ammonia. So, the final order is -
Question 13.4(ii) Arrange the following in increasing order of their basic strength:
Answer :
On considering the inductive effect, solvation effect and steric hindrance of the alkyl group the increasing order of basicity in ethyl as a substituted group is shown above.
Question 13.4(iii) Arrange the following in increasing order of their basic strength:
Answer :
On considering the inductive effect, solvation effect and steric hindrance of the alkyl group which decides the basic strength of alkyl amines in the aqueous state. The order of basic strength in case of methyl substituted amines are -
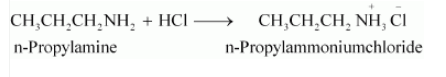
Question 13.5(i) Complete the following acid-base reactions and name the products:
Answer :
The above-given reaction is an acid-base reaction. so salt is formed.
Question 13.5(ii) Complete the following acid-base reactions and name the products:
Answer :
It is an acid-base reaction, so the N will accept H from and form a salt.
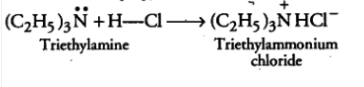
Answer :
The methyl iodide reacts with aniline to give N, N-dimethylaniline.
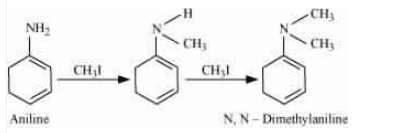 With the excess of methyl iodide in the presence of sodium carbonate solution (
With the excess of methyl iodide in the presence of sodium carbonate solution ( ), N, N-dimethylaniline produces N, N, N-trimethyl anilinium carbonate.
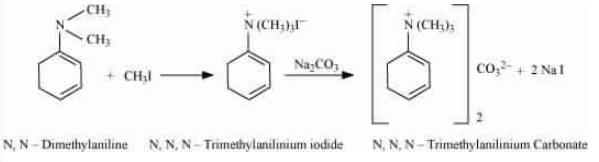
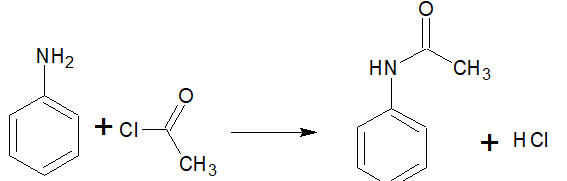
Question 13.7 Write chemical reaction of aniline with benzoyl chloride and write the name of the product obtained.
Answer :
When aniline reacts with benzoyl chloride HCl is produced as a by-product and N-phenyl benzamide is produced as a major product.
lone pair of N atom attacks the acidic carbon of benzoyl chloride.
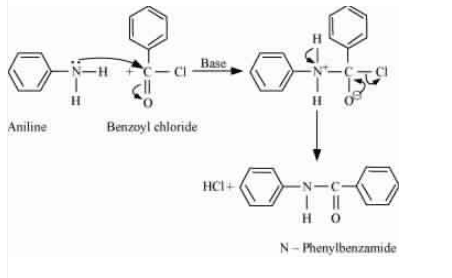
Answer :
structures of different isomers corresponding to the molecular formula and their IUPAC name-
(i) | 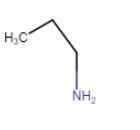 | Propan-1-amine |
(ii) | 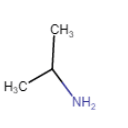 | Propan-2-amine |
(iii) | 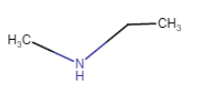 | N – Methylethanamine |
(iv) | 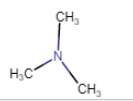 | N, N-Dimethylmethanamine |
The structure (i) and (ii) will liberate nitrogen gas ( ) on treating with nitrous acid.
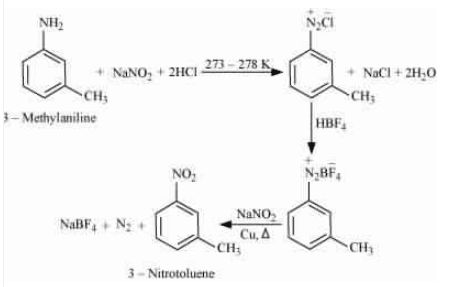
Question 13.9(i) Convert
3-Methylaniline into 3-nitrotoluene
Answer :
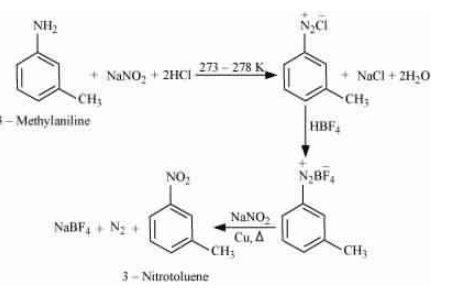
On diazotisation, reaction will convert to
. And then reacts with fluoroboric acid followed by
convert
into the nitro group (
) and evolution of dinitrogen gas.
Question 13.9(ii) Convert
Aniline into 1,3,5 - tribromobenzene.
Answer :
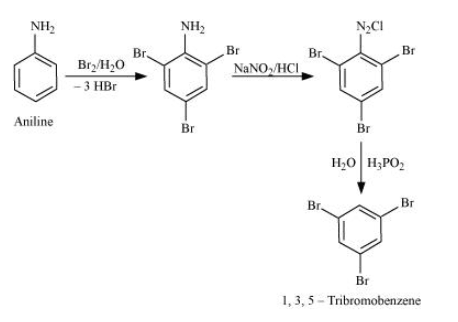 Aniline on bromination in the presence of water gives 2,4,6 tribromoaniline, which on further reacting with nitrous acid converts
Aniline on bromination in the presence of water gives 2,4,6 tribromoaniline, which on further reacting with nitrous acid converts into
. Now, with the help of
it removes the
group from the benzene ring and we get our final product 1,3,5 tribromobenzene.
NCERT Solutions for Class 12 Chemistry Chapter 13 Amines- Exercise Questions
Question 13.1 Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer :
The IUPAC name of the compound is 1-methylethanamine.
since (nitrogen ) is connected to only one carbon. Hence it is a primary amine.
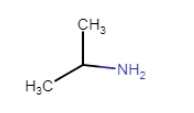
structure of the compound
Question 13.1(ii) Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer :
IUPAC name is Propan-1-amine
It is a primary amine (nitrogen connects with only one carbon atom)
Here is the structure of the compound -
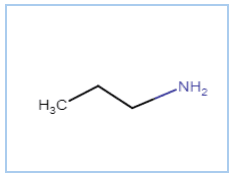
Question 13.1 (iii) Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer :
N-Methyl-2-methylethanamine
since N atom is connected with two C atom, So it is a two-degree amine
Question 13.1(iv) Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer :
2-Methylpropane-2-amine
It is a one-degree amine
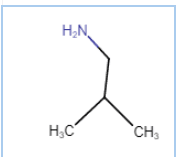
Question 13.1(v) Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer :
N-Methylbenzamine or N-methylaniline
Here N atom is connected with two C atom. So, it is a secondary amine.
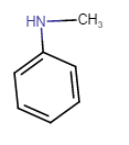
Question 13.1(vi) Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer:
N-Ethyl-N-methylethanamine
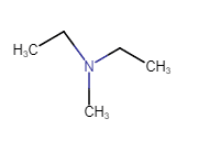
N is connected with three carbon atom so that it is a tertiary amine
Question 13.1(vii) Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer :
3-bromoaniline or 3-bromobenzenamine
It is a primary amine
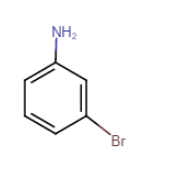
Question 13.2 Give one chemical test to distinguish between the following pairs of compounds.
(i) Methylamine and dimethylamine
Answer :
Carbylamine test
This test is used to distinguish between aliphatic and aromatic primary amines on heating with chloroform and potassium hydroxide (
) gives isocyanides or carbylamines which has foul smelling. two degree and 3-degree amines do not show this reaction.
Here methylamine is primary and dimethylamine is a secondary amine.
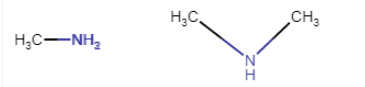
first one is 1-degree and second one is 2-degree
Question 13.2 Give one chemical test to distinguish between the following pairs of compounds.
(ii) Secondary and tertiary amines
Answer :
Secondary and tertiary amines can be distinguished by reacting them with Hinsberg's reagent which is also called benzenesulphonyl chloride. In the case of primary amine the product is soluble in alkali but not in secondary amine case. And tertiary amine does not react with this reagent.
In the case of a secondary amine
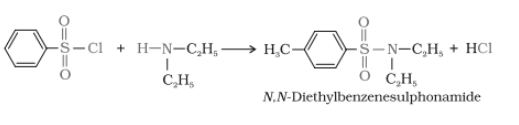
Tertiary amine + benzenesulphonyl chloride no reaction
Question 13.2 Give one chemical test to distinguish between the following pairs of compounds.
Answer :
Ethylamine and aniline can be differentiated by the azo-dye test. On reacting with and followed by 2-naphthol gives a colored product. But when it is ethylamine it gives brisk effervescence due to the evolution of
gas under the same conditions.
Question 13.2 Give one chemical test to distinguish between the following pairs of compounds.
Answer :
Benzylamine reacts with nitrous acid to form diazonium salt, which is unstable and also gives alcohol with the evolution of nitrogen gas. On the other hand, aniline reacts with nitrous acid to form a stable diazonium salt.
Question 13.2 Give one chemical test to distinguish between the following pairs of compounds.
(v) Aniline and N-methylaniline.
Answer :
Aniline and N-methylaniline both can be distinguished by carbylamine test. aniline is the primary aromatic compound so it gives s positive test but N-methyl aniline is secondary and does not give this test.
structure of both compound-
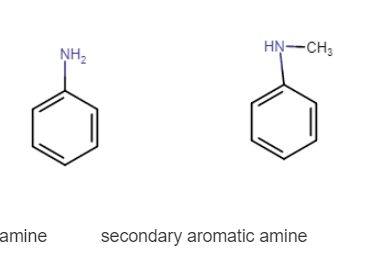 primary aromatic amine secondary aromatic amine
primary aromatic amine secondary aromatic amine
13.2 Give one chemical test to distinguish between the following pairs of compounds. (v) Aniline and N-methylaniline.
Edit Q
Question 13.3 Account for the following:
(i) pKb of aniline is more than that of methylamine..
Answer :
Here is the structure of aniline and methylamine
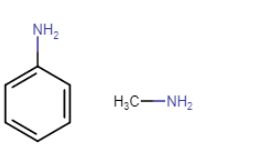
Due to the resonance in aniline the electrons of nitrogen atoms delocalised over benzene ring. So, because of that, the electron density at N atom decreases and less available for donation.
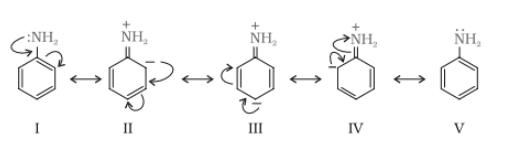 On the other hand, In the case of methylamine, the methyl (
On the other hand, In the case of methylamine, the methyl ( ) group is electron donating group (+I effect) which increase the electron density at N atom. Hence
value of aniline is higher than that of methylamine.
Question 13.3 Account for the following:
(ii) Ethylamine is soluble in water whereas aniline is not.
Answer:
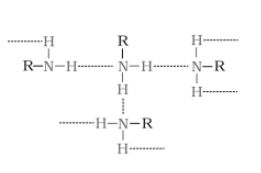
The extent oh intermolecular hydrogen bonding in ethylamine is very high. Hence it is soluble in water. But aniline does not undergo hydrogen bonding with water to a large extent due to the presence of a bulky hydrophobic group . Hence it is insoluble in water.
Question 13.3 Account for the following:
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
Answer :

Due to +I effect of methyl ( ) group methylamine is more basic than water. So in water
produces hydroxide ion by accepting
ions from water.
Ferric chloride ( ) dissociates in water into-
The hydroxide ion (
) react with
and form a precipitate of
(reddish brown ppt)
Question 13.3 Account for the following:
Answer :
Nitration is carried out in strongly acidic medium, aniline is protonated to form the anilinium ion which is meta directing. Because of this reason, aniline on nitration gives a substantial amount of meta - nitroaniline 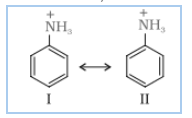
Question 13.3 Account for the following:
(v) Aniline does not undergo Friedel-Crafts reaction.
Answer :
In fridal craft reaction, we use which is acidic in nature and aniline is also a show basic nature. Thus there is an acid-base reaction between them and form a salt.
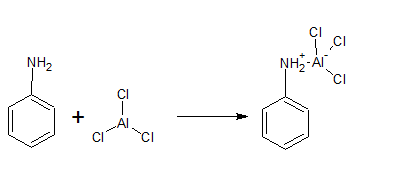
Due to the positive charge on the N-atom. the electrophilic substitution in benzene ring is deactivated.
Question 13.3 Account for the following:
(vi) Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Answer :
In diazonium salt, the structure goes under resonance due to which the dispersal of positive charge is more and we know that higher is the resonance higher is the stability. Therefore diazonium salt of aromatic amines is more stable than those of aliphatic amines.
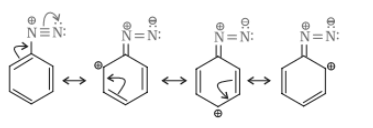
Question 13.3 Account for the following:
(vii) Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Answer :
Gabriel synthesis is used for primary amines. secondary and tertiary amines are not formed by this method. So, therefore to obtain pure and only 1-degree amine Gabriel phthalimide reaction is preferred.
Question 13.4 Arrange the following:
(i) In decreasing order of the pKb values:
Answer :
and
are aliphatic amines so they are more basic than rest two compound. In ethylamine, there is only one electron donating group (+I effect) but in diethylamine, there is two electron donating group so the electron density is much higher than ethylamine.
In between and
they are the weak base because of delocalisation of lone pair electron in the benzene ring. Aniline is less basic than N-methylaniline(
) due to the absence of an electron donating group. Thus the decreasing order of pKb values is-
Question 13.4 Arrange the following:
(ii) In increasing order of basic strength:
Answer :
The increasing order of basic strength-
Aliphatic amines are more basic than aromatic amines due to the presence of high electron density of electron at N atom so that it can donate an electron. On the other hand in aromatic amines the lone pair of electron delocalised in the benzene ring so the availability of electron is less.
is more basic because of +I effect of the two ethyl group (so electron density is highest in this compound) while methylamine has one ethyl group. In aromatic amines, diethylaniline is more basic due to the presence of more number of electron donating group.
Question 13.4 Arrange the following:
(iii) In increasing order of basic strength:
(a) Aniline, p-nitroaniline and p-toluidine
Answer :
Structure of the following compounds-
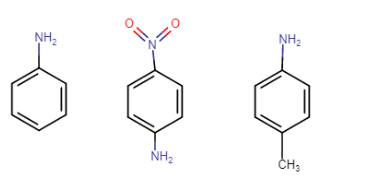 Aniline, p-nitroaniline p-toluidine
Aniline, p-nitroaniline p-toluidine
In p-toluidine, the methyl group increases the electron density at the N atom (+I effect of methyl), so it is more basic than aniline. In p-nitroaniline, the nitro group is an electron withdrawing group, so it decreases the electron density from N atom, so it is less basic than aniline.
Question 13.4 Arrange the following:
(iii) In increasing order of basic strength:
Answer :
The increasing order of basic strength-
it is more basic than aniline due to the presence of one electron donating group which increases the electron density at N atom. And also in this compound N atom is directly attached with the benzene ring, so due to -R effect
is less basic than
Question 13.4 Arrange the following:
(iv) In decreasing order of basic strength in gas phase:
Answer :
Decreasing order of basic strength in the gas phase-
In the gas phase, there is no solvation effect. So, the basicity directly depends on the number of electron donation group. More is the electron donating group, higher is the +I effect and if the size of the donating group is large +I effect should also high.
Question 13.4 Arrange the following:
(v) In increasing order of boiling point:
Answer :
The boiling point of the compounds depends on the extent of hydrogen bonding of the compound. More is the extent; higher is the boiling point. , it contains two H atoms, so it has a higher boiling point than
. On the other hand, Oxygen atom is more electronegative than N atom, so the strength of hydrogen bonding is higher in
than
THus the increasing order of boiling point is-
Question 13.4 Arrange the following:
(vi) In increasing order of solubility in water:
Answer :
More extensive is the hydrogen bonding, more is the solubility in water. contains two H atom while
contains only one. So, the
undergoes extensive hydrogen bonding. Hence its solubility is more than
in water. On increasing the molecular mass of amines decreases its solubility in water because of an increase in bulky hydrophobic groups.
Thus the increasing order of solubility in water is-
Question 13.5 How will you convert:
(i) Ethanoic acid into methanamine
Answer :
Ethanoic acid on reacting with replaces the OH by
and then reacts it with an excess of ammonia molecule to produce methanamide (
) which by Hoffmann bromamide degradation reaction gives us desired product (methenamine)
Question 13.5 How will you convert:
(ii) Hexanenitrile into 1-aminopentane
Answer :
First, acid hydrolysis of hexanenitrile which convert CN group into COOH group and then react with which replace COOH by
and then the excess of ammonia which replace Cl by
and then Hoffmann bromamide degradation reaction which gives us desired product.
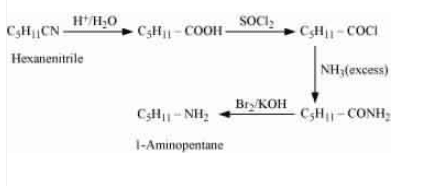
Question 13.5 How will you convert:
(iii) Methanol to ethanoic acid
Answer :
methanol on reacting with phosphorous pentachloride, OH is reduced by Cl to form chloromethane, which on reacting with ethanolic sodium cyanide gives followed by acidic hydrolysis it produces ethanoic acid.(
)
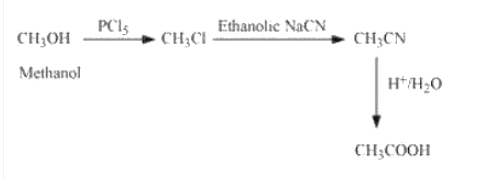
Question 13.5 How will you convert:
(iv) Ethanamine into methanamine
Answer :
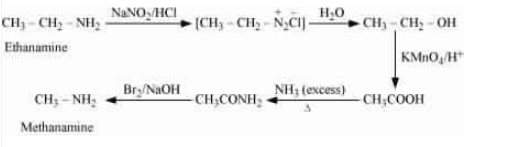
React ethanamine with nitrous acid to form an azo compound, which further reacts with water to form ethanol, which on oxidising gives ethanoic acid. After treating with an excess of ammonia the ethanoic acid becomes ethanamide, which on further reacting with Bomine and a strong base (Hoffmann bromamide degradation reaction) to form methenamine.
Question 13.5 How will you convert:
(v) Ethanoic acid into propanoic acid
Answer :
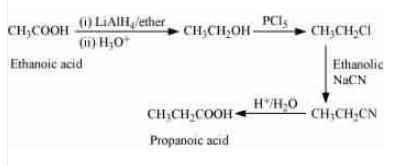
Acetic acid on reduction with lithium aluminium hydride followed by acid hydrolysis gives ethanol, which on reacting with gives ethyl chloride. Ethyl chloride on further reacting with ethanolic sodium cyanide to form propanenitrile, which on acidic hydrolysis gives the desired output.
Question 13.5 How will you convert:
(vi) Methanamine into ethanamine
Answer :
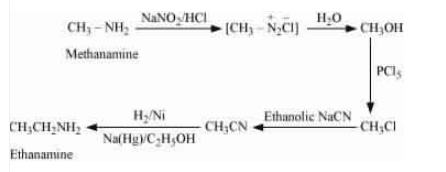
Methenamine on diazotisation gives methane diazonium salt, which on further hydrolysis form methanol. Methanol on reacting with followed by ethanolic sodium cyanide (
) produces Ethane nitrile, which on reduction with
gives ethenamine.
Question 13.5 How will you convert:
(vii) Nitromethane into dimethylamine
Answer :
Conversion of Nitromethane into dimethylamine is shown below:
Question 13.5 How will you convert:
(viii) Propanoic acid into ethanoic acid?
Answer :
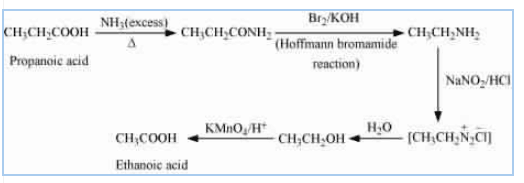
![]()
Propanoic acid on reacting with an excess of ammonia gives propanamide which on further react with bromine and potassium hydroxide (Hoffmann bromamide degradation reaction) gives ethenamine. On diazotisation, ethenamine converted into azo salt which on treating with water forms ethanol. Ethanol on oxidation provides ethanoic acid.
Answer :
Primary, secondary and tertiary amines are distinguished by the Hinsberg's reagent test. In this test, the amines are allowing to react with benzene sulphonyl chloride(Hinsberg's reagent). All three types of amines give different products.
1.Primary amine reacts with benzene sulphonyl chloride gives N-ethylbenzene sulphonamide which is soluble in alkali.
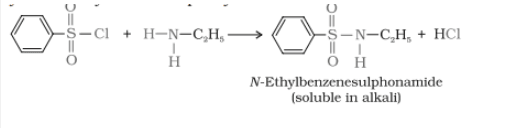
2. when secondary amines react it gives N, N-diethyl benzene sulphonamide which is insoluble in alkali.
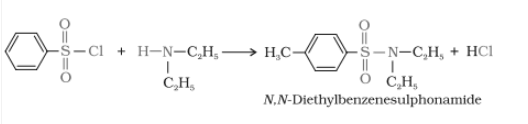
3. Tertiary amine does not react with Hinsberg's reagent.
Question 13.7 Write short notes on the following:
Answer :
Carbylamine reaction-
Aliphatic and aromatic primary amines on reacting with chloroform and ethanolic KOH form isocyanides or carbylamine which is foul smelling substance. Secondary and tertiary amine does not give this reaction. This reaction is known as carbylamine reaction or isocyanide test. It is used to distinguish primary amine.
Question 13.7 Write short notes on the following:
Answer :
DIAZOTISATION-
Aromatic primary amines react with nitrous acid ( )at low temperature to form diazonium salts. This conversion of aromatic primary amines into diazonium salt is known as diazotisation.
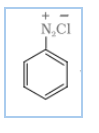
![]()
Question 13.7 Write short notes on the following:
(iii) Hofmann’s bromamide reaction
Answer :
Hofmann’s bromamide reaction-
When an amide is treated with bromine in aqueous solution or ethanolic solution of sodium hydroxide. It produces primary amines with one less carbon atom than the parent compound. This reaction is known as the Hoffmann bromide degradation reaction.
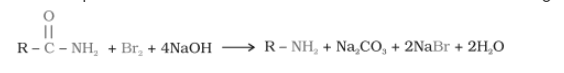 R = alkyl group like
R = alkyl group like
Question 13.7 Write short notes on the following:
Answer :
Coupling reaction-
The reaction of joining of diazonium salt to the aromatic ring (like phenol) through bond is known ass coupling reaction. Benzene diazonium chloride reacts with phenol in which the phenol molecule at its para position is coupled with the diazonium salt(
) to give p-hydroxyazobenzene(orange in colour)
Reactions-
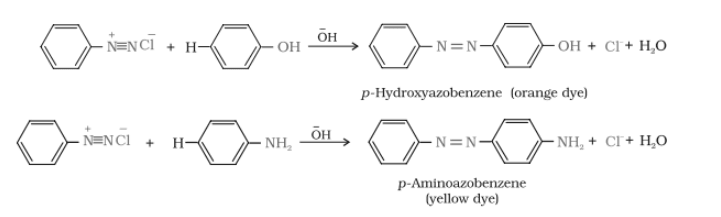 diazonium salt reacts with aniline to give p-aminoazobenzene (yellow colour)
diazonium salt reacts with aniline to give p-aminoazobenzene (yellow colour)
Question 13.7 Write short notes on the following:
Answer :
Ammonolysis-
When an alkyl or benzyl halide is going to react with the solution of ammonia(an ethanolic) undergoes nucleophilic substitution reaction in which the halogen atom is replaced by an amino ( ) group. This process of cleavage of the carbon-halogen(C-X) bond by ammonia molecule is known as ammonolysis.
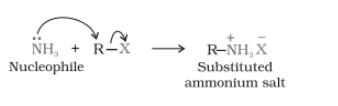 When this substituted ammonium salt is treated with a strong base it gives primary amine
When this substituted ammonium salt is treated with a strong base it gives primary amine
- Above primary amine is behaves as a nucleophile and can further react with an alkyl halide to form secondary and tertiary amines and finally quaternary ammonium salt.
-
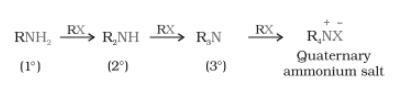
Question 13.7 Write short notes on the following:
Answer :
Acetylation-
The introduction of an acetyl group ( )in a molecule is known as acetylation.
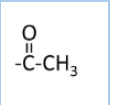
![]()
Example-
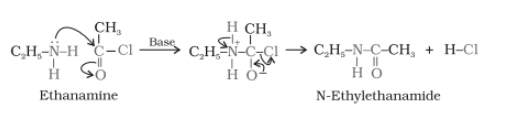 Aliphatic and aromatic primary and secondary amines undergo acetylation reaction when treated with acid chlorides, anhydrides or esters by nucleophilic substitution. Here Hydrogen atom of
Aliphatic and aromatic primary and secondary amines undergo acetylation reaction when treated with acid chlorides, anhydrides or esters by nucleophilic substitution. Here Hydrogen atom of or
is replaced by
the group.
Question 13.7 Write short notes on the following:
(vii) Gabriel phthalimide synthesis.
Answer :
Gabriel phthalimide synthesis.-
It is used for mainly aliphatic primary amine preparation. Phthalimide reacts with ethanolic potassium hydroxide and produces potassium salt of phthalimide which on heating with alkyl halide followed by alkaline hydrolysis gives corresponding primary amine. Aromatic amines cannot be produced by this method due to the inability of aryl halides to do nucleophilic substitution reaction with anion produced by phthalimide.
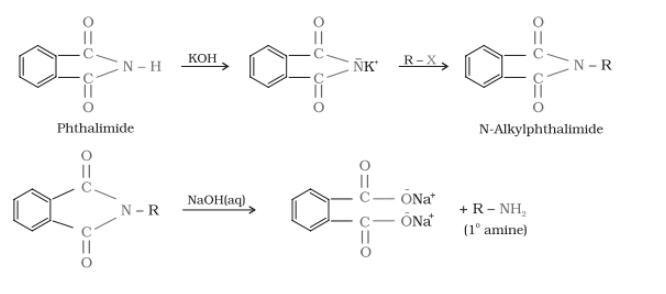
Question 13.8 Accomplish the following conversions:
(i) Nitrobenzene to benzoic acid
Answer :
First, reduce nitrobenzene into aniline with the help of . Now convert aniline to diazonium salt followed by sandmeyers reaction (
) to benzonitrile and after acid hydrolysis benzonitrile becomes benzoic acid.
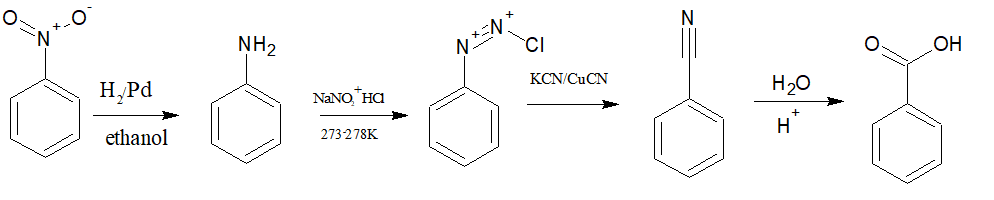
Question 13.8 Accomplish the following conversions:
Answer :
First, We do nitration of benzene so that it can direct the bromine to meta position and then bromination of nitrobenzene after that reduce it with the help of followed by diazotisation reaction and then hydrolysis in warm water.
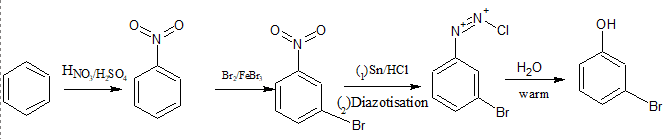
Question 13.8 Accomplish the following conversions:
Answer :
On reacting benzoic acid with we get benzene sulphonyl chloride and when it reacts with ammonia the chlorine is replaced by
and after that do Hoffmann bromide degradation reaction to remove -CO group.

Question 13.8 Accomplish the following conversions:
(iv) Aniline to 2,4,6 -tribromofluorobenzene
Answer :
First, we do bromination of aniline which gives 2,4,6-tribromoaniline after that do diazotisation reaction which converts group into
and then react it with fluoroboric acid(
) gives us desired product.
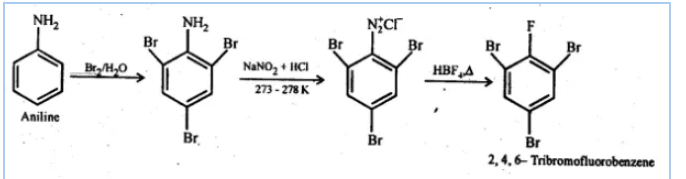
![]()
Question 13.8 Accomplish the following conversions:
(v) Benzyl chloride to 2-phenylethanamine
Answer :
On reacting benzyl chloride with the ethanolic sodium cyanide ( ), it replaces the Chlorine with CN molecule. And then reduction with the help of
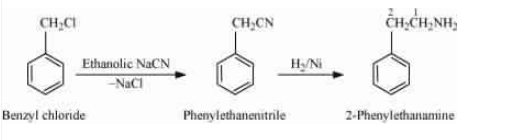
Question 13.8 Accomplish the following conversions:
Answer :
On nitration of chlorobenzene, it gives p-nitrochlorobenzene which is on further reacting with , it reduces
to

Question 13.8 Accomplish the following conversions:
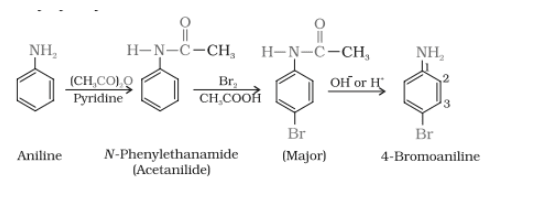
Answer :
Aniline on reacting with the acetic anhydride in the presence of pyridine it gives N-phenylethanamide which on further reacting with the
group direct the bromine to the para- position and after hydrolysis, we can recover the original
group at the same position
Question 13.8 Accomplish the following conversions:
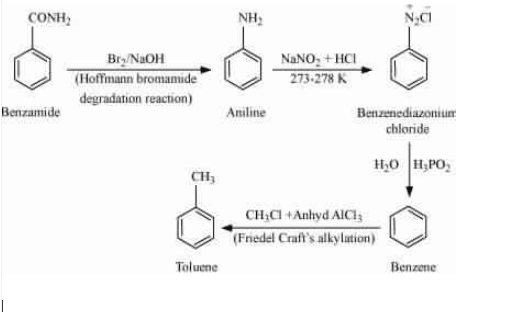
Answer :
Use the Hoffmann bromamide degradation reaction so that it gives aniline and then do diazotisation reaction so that group is replaced by
and then reduction with the help of (
) so that it reduced into the benzene ring. And then alkylation of benzene gives toluene as a final product.
Question 13.8 Accomplish the following conversions:
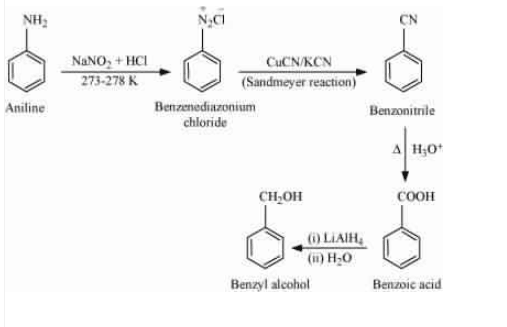
(ix) Aniline to benzyl alcohol
Answer :
Aniline on treating with nitrous acid followed by sandmeyers reaction gives benzonitrile ( ), which on acidic hydrolysis form benzoic acid (
) and then reduction process with
(lithium aluminium hydride) to get desired product.
![]()
Question 13.9 Give the structures of A, B and C in the following reactions:
Answer :
In the first reaction, the iodine is replaced by CN so the structure of (A) is ( propionitrile) . On partial hydrolysis, the CN converted in to
, So the structure of B is
(propanamide) and the third reactant is Hoffmann bromide degradation reaction, So just remove the -CO group and it becomes a primary aliphatic amine. Thus the structure of C is
(ethanamine)
Question 13.9 Give the structures of A, B and C in the following reactions:
Answer :
The first part is the Sandmeyers reaction so CN replaces the of the reactant and the product (A) is cynobenzene
. On acidic hydrolysis of cynobenzene give product (B) benzoic acid
and benzoic acid react with ammonia (heat) it produces product (C)benzamide
The structure of A, C and B are-

Question 13.9 Give the structures of A, B and C in the following reactions:
Answer :
The structure of A, B, C are respectively and
In the first reaction, the bromine is replaced by cyanide molecule ( ) and after a reduction by
the CN, molecules become
.
Question 13.9 Give the structures of A, B and C in the following reactions:
Answer :
A-
B-
C-
The First nitrobenzene reduced to aniline and then it reacts with to form benzene diazonium salt and is hydrolysed to form phenol(
)
Question 13.9 Give the structures of A, B and C in the following reactions:
Answer :
A- ethanamide ( )
B- methenamine ( )
C- methanol ( )
Acetic acid reacts with ammonia, gives ethanamide and the second reaction is Hoffmann bromamide degradation reaction so it removes -CO group and form methenamine and after that the last product is methanol.
Question 13.9 Give the structures of A, B and C in the following reactions:
Answer :
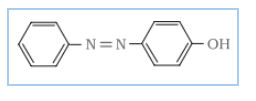
Initially, product A is the reduction of nitrobenzene means it is an aniline. when A react with it gives benzene diazonium chloride(B). And when B reacts with another aromatic ring (phenol) it's a coupling reaction so it gives azodye (p-Hydroxyazobenzene)
A= Aniline B = benzene diazonium chloride C= p-Hydroxyazobenzene
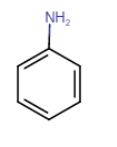
![]()
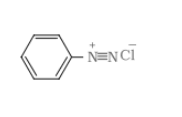
Answer :
The molecular formula of C =
it is a Hoffmann bromamide degradation reaction so, the product should be
(aniline)
So B should be benzamide ( ) and A should be benzoic acid (
)
Therefore the above reaction proceeds as-

Question 13.11 Complete the following reactions:
Answer :
The given above reaction is carbylamine reaction in which primary aromatic amines react with chloroform and alc. potassium hydroxide to give isocyanide or carbylamine as a product which is foul-smelling substance.
Question 13.11 Complete the following reactions:
Answer :
When benzene diazonium chloride reacts with it reduced into benzene and
oxidised into
, HCl produced as a by-product in this reaction.
Question 13.11 Complete the following reactions:
Answer :
Aniline is a base and is acid. So, basically, it is acid-base reaction aniline accepts
ions from sulphuric acid and forms a product anilinium hydrogen sulphate.
Question 13.11 Complete the following reactions:
Answer :
Ethanol reduces the benzene diazonium chloride to benzene and oxidises into ethanal ( ), hydrochloric acid produces as a by-product and evolution of nitrogen gas is also occurring.

Question 13.11 Complete the following reactions:
Answer :
Aniline on bromination in the presence of water at room temperature gives a white ppt of 2, 4, 6- tribromoaniline
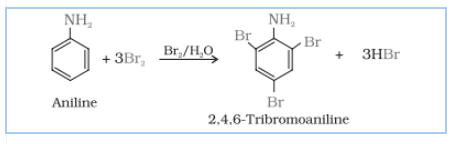
Question 13.11 Complete the following reactions:
Answer :
Aniline reacts with acetic anhydride by a nucleophilic substitution reaction. Here the replacement of H atom from group is by an acyl group. The product formed is N-phenylethanamide.p. The reaction is carried out in the presence of a base stronger than the amine, like pyridine
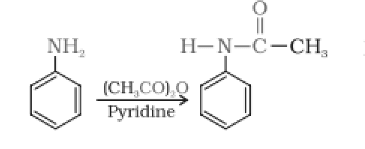
Question 13.11 Complete the following reactions:
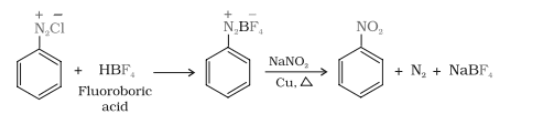
Answer :
In this reaction, the chloride ion is replaced by ion. When diazonium fluoroborate is heated with aqueous sodium nitrite in the presence of copper, the diazonium group is replaced by –NO2 group.
Question 13.12 Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
Answer :
Gabriel phthalimide synthesis is used for mainly aliphatic primary amine preparation. Phthalimide reacts with ethanolic potassium hydroxide and produces potassium salt of phthalimide
which on heating with alkyl halide followed by alkaline hydrolysis gives corresponding primary amine.
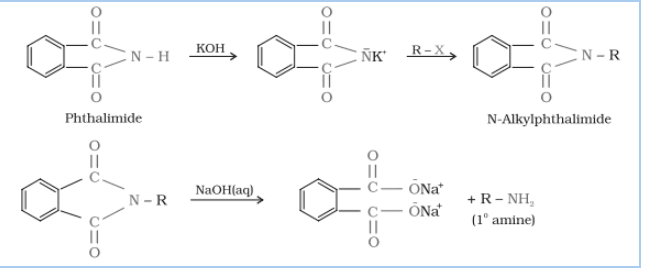
![]()
Aromatic amines cannot be produced by this method due to the inability of aryl halides to do nucleophilic substitution reaction with anion produced by phthalimide.
Hence aromatic amines cannot be produced by this method.
{if R = Aromatic compound}
Question 13.13 Write the reactions of
(i) aromatic primary amines with nitrous acid.
Answer :
Aromatic primary amines react with nitrous acid( ) at 273-278 K to form stable diazonium salts. aniline reacts with nitrous acid to form benzene diazonium chloride.
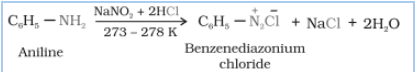
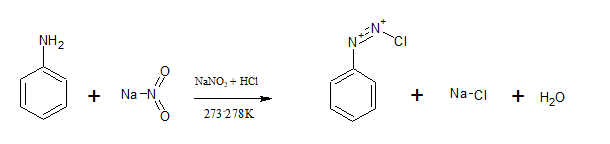
Question 13.13 Write the reactions of
(ii) aliphatic primary amines with nitrous acid.
Answer :
When aliphatic primary amines react with nitrous acid to form unstable diazonium salts which on further produces alcohol and hydrochloric acid ( ) with the evolution of dinitrogen gas (
).
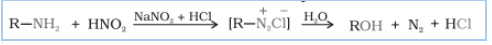
Question 13.14 Give a plausible explanation for each of the following:
(i) Why are amines less acidic than alcohols of comparable molecular masses?
Answer :
Protonation of amines gives- (amide ion)
protonation of alcohol gives - (alkoxide ion)
the negative charge is easily accommodated by O atom because it is more electronegative than N atom. So, the amide ion is less stable than the alkoxide ion. Hence amines are less acidic than alcohols.
Question 13.14 Give a plausible explanation for each of the following:
(ii) Why do primary amines have a higher boiling point than tertiary amines?
Answer :
The extent of inter-molecular hydrogen bonding in primary amines is higher than the secondary amines. Secondary amine has only one hydrogen, and tertiary amines have no hydrogen atom for inter-molecular hydrogen bonding whereas primary amine has two hydrogen atom available for inter-molecular hydrogen bonding. And we know that higher the extent of hydrogen bonding, higher is the boiling point.
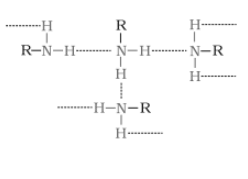 (In
(In amine),
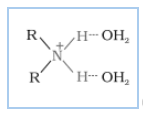 (In
(In amines),
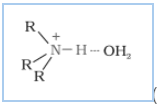 (In
(In amine)
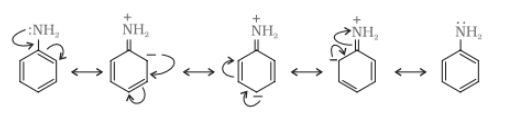
Question 13.14 Give plausible explanation for each of the following:
(iii) Why are aliphatic amines stronger bases than aromatic amines?
Answer :
Aliphatic amines stronger bases than aromatic amines due to resonance in aromatic base the lone pair of electron of N atom delocalised over the benzene ring, which makes it less available for electron donation. On the other hand, in aliphatic amines( ), the electron donor group is attached which increase the electron density at N atom. Thus the aliphatic amines are more basic than aromatic amines.
Features of Amines Class 12 NCERT Chapter
In NCERT solutions for class 12 chemistry chapter 13 Amines, there are ten sub-topics from which questions are generally asked that covers important concepts of chemistry such as the structure, nomenclature, and classification of amines and also covers its chemical and physical properties and method of preparation of diazonium salts. After studying NCERT solutions for Class 12 chemistry chapter 13 Amines you will be able to describe amines as derivatives of ammonia; classify amines as primary, secondary and tertiary; explain IUPAC nomenclature of amines; describe methods of preparation of amines and properties of amines.
Topics and Sub-topics of Amines Class 12 NCERT Chapter
13.1 Structure of Amines
13.2 Classification
13.3 Nomenclature
13.4 Preparation of Amines
13.5 Physical Properties
13.6 Chemical Reactions
13.7 Method of Preparation of Diazonium Salts
13.8 Physical Properties
13.9 Chemical Reactions
13.10 Importance of Diazonium Salts in the Synthesis of Aromatic Compounds
NCERT Solutions for Class 12 Chemistry
Chapter 1 | |
Chapter 2 | |
Chapter 3 | |
Chapter 4 | |
Chapter 5 | |
Chapter 6 | |
Chapter 7 | |
Chapter 8 | |
Chapter 9 | |
Chapter 10 | |
Chapter 11 | |
Chapter 12 | |
Chapter 13 | NCERT solutions for class 12 chemistry chapter 13 Amines |
Chapter 14 | |
Chapter 15 | |
Chapter 16 |
NCERT Solutions for Class 12 Subject wise
Benefits of NCERT Solutions for class 12 chemistry chapter 13 Amines
- The answers presented in a step-by-step manner in the NCERT solutions for class 12 chemistry chapter 13 Amines will help in understanding chapter easily.
- It will be easy to revise because the detailed solutions will help you to remember the concepts and fetch you good marks.
- Homework problems won't bother you anymore, all you need to do is check the detailed NCERT solutions for class 12 chemistry chapter 13 Amines and you are ready to sail.
SAT® | CollegeBoard
Registeration closing on 19th Apr for SAT® | One Test-Many Universities | 90% discount on registrations fee | Free Practice | Multiple Attempts | no penalty for guessing
TOEFL ® Registrations 2024
Thinking of Studying Abroad? Think the TOEFL® test. Save 10% on your TOEFL exam with ApplyShop gift cards!
If you have a doubt or question that is not available here or in any of the chapters, contact us. You will get all the answers that will help you score well in your exams.
Also check NCERT Exemplar Class 12 Solutions
- NCERT Exemplar Class 12th Maths Solutions
- NCERT Exemplar Class 12th Physics Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Class 12th Biology Solutions
Also Check NCERT Books and NCERT Syllabus here:
Frequently Asked Question (FAQs)
Around 5-7 marks worth questions are asked from this chapter in JEE mains. The questions for Amines can be practiced from the NCERT book, NCERT exemplar and JEE Main previous year papers.
For complete solutions : https://school.careers360.com/ncert/ncert-solutions-class-12-chemistry. Students can download the chapter wise solutions PDF.
This chapter holds weightage of 5-6 Marks in Board exams. Follow NCERT syllabus to get a good score in the CBSE board exam.
Weightage of NCERT class 12 Chemistry chapter 13 in NEET is around 3 percent
Also Read
Articles
Certifications By Top Providers
Explore Top Universities Across Globe
Questions related to CBSE Class 12th
Hello aspirant,
The purpose of graphic design extends beyond the brand's look. Nevertheless, by conveying what the brand stands for, it significantly aids in the development of a sense of understanding between a company and its audience. The future in the field of graphic designing is very promising.
There are various courses available for graphic designing. To know more information about these courses and much more details, you can visit our website by clicking on the link given below.
https://www.careers360.com/courses/graphic-designing-course
Thank you
Hope this information helps you.
hello,
Yes you can appear for the compartment paper again since CBSE gives three chances to a candidate to clear his/her exams so you still have two more attempts. However, you can appear for your improvement paper for all subjects but you cannot appear for the ones in which you have failed.
I hope this was helpful!
Good Luck
Hello dear,
If you was not able to clear 1st compartment and now you giving second compartment so YES, you can go for your improvement exam next year but if a student receives an improvement, they are given the opportunity to retake the boards as a private candidate the following year, but there are some requirements. First, the student must pass all of their subjects; if they received a compartment in any subject, they must then pass the compartment exam before being eligible for the improvement.
As you can registered yourself as private candidate for giving your improvement exam of 12 standard CBSE(Central Board of Secondary Education).For that you have to wait for a whole year which is bit difficult for you.
Positive side of waiting for whole year is you have a whole year to preparing yourself for your examination. You have no distraction or something which may causes your failure in the exams. In whole year you have to stay focused on your 12 standard examination for doing well in it. By this you get a highest marks as a comparison of others.
Believe in Yourself! You can make anything happen
All the very best.
Hello Student,
I appreciate your Interest in education. See the improvement is not restricted to one subject or multiple subjects and we cannot say if improvement in one subject in one year leads to improvement in more subjects in coming year.
You just need to have a revision of all subjects what you have completed in the school. have a revision and practice of subjects and concepts helps you better.
All the best.
Hi,
You just need to give the exams for the concerned two subjects in which you have got RT. There is no need to give exam for all of your subjects, you can just fill the form for the two subjects only.
Popular CBSE Class 12th Questions
Colleges After 12th
Also Read
Applications for Admissions are open.

JEE Main Important Physics formulas
ApplyAs per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

UPES School of Liberal Studies
ApplyRanked #52 Among Universities in India by NIRF | Up to 30% Merit-based Scholarships | Lifetime placement assistance

Aakash iACST Scholarship Test 2024
ApplyGet up to 90% scholarship on NEET, JEE & Foundation courses

JEE Main Important Chemistry formulas
ApplyAs per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

PACE IIT & Medical, Financial District, Hyd
ApplyEnrol in PACE IIT & Medical, Financial District, Hyd for JEE/NEET preparation

ALLEN JEE Exam Prep
ApplyStart your JEE preparation with ALLEN