Apply to Aakash iACST Scholarship Test 2024
NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry
NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry- Electrochemistry is the study of the interconversion of chemical energy and electrical energy. The devices where these conversions take place are known as cells. NCERT solutions for Class 12 Chemistry chapter 3 Electrochemistry deal with questions based on mainly electrochemical and galvanic cells and also on Nernst equation in order to calculate electromotive force potential. Electrochemistry Class 12 will also acknowledge you to various types of batteries and their benefits. The chapter is important for both theoretical and practical purposes. therefor electrochemistry class 12 NCERT solutions become very important to get in-depth understanding of concepts.
Latest: JEE Main high scoring chapters
Don't Miss: JEE Main 10 year's papers
Recommended: NEET high scoring chapters
Suggested: NEET question papers with solutions
Also Read,
Important points and formulas of NCERT Class 12 Chemistry Chapter 3 Electrochemistry-
1. Conductance(G) is the reciprocal of resistance (R) and specific conductance or conductivity(k) is inverse of resistivity
2. l/a is called the cell constant of conductivity cell.
3. Equivalent Conductivity is defined as the conductance of a solution containing 1g of an electrolyte.
4. Nernst equation
aA+bB cC+dD
class 12 chemistry electrochemistry ncert solutions
Topics and Sub-topics of NCERT Electrochemistry Class 12 Chemistry Chapter 3 -
3.1 Electrochemical Cells
3.2 Galvanic Cells
3.3 Nernst Equation
3.4 Conductance of Electrolytic Solutions
3.5 Electrolytic Cells and Electrolysis
3.6 Batteries
3.7 Fuel Cells
3.8 Corrosion
Find Solutions of NCERT Class 12 Chemistry Chapter 3 Electrochemistry
Solutions to In-Text Questions Ex 3.1 to 3.15
Question 3.1 How would you determine the standard electrode potential of the system ?
Answer :
To determine the standard electrode potential of the given system we need to use a hydrogen electrode. In the setup, we shall put a hydrogen electrode as cathode and Mg | MgSO 4 as an anode.
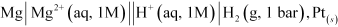 Now we will measure the emf of the cell. This emf will be the standard electrode potential of the magnesium electrode.
Now we will measure the emf of the cell. This emf will be the standard electrode potential of the magnesium electrode.
E°cell = E° right – E°left
E°left =0 ( The standard hydrogen electrode is always zero)
Hence
Question 3.2 Can you store copper sulphate solutions in a zinc pot?
Answer:
The standard electrode potential of Zinc is - 0.76 whereas that of Copper is 0.34. So Zinc will reduce copper into the lower state.
It is known that zinc is more reactive than copper. Thus if we will store copper sulphate solution in zinc pot then zinc will displace copper from its solution.
The following reaction will take place:-
Answer :
The oxidising strength of elements increases as the standard electrode potential increases.
So all the elements having greater standard potential than iron can oxidise it to a higher state.
Few such elements are :- F 2 , Cl 2 , Br 2 , Ag +1 etc.
Question 3.4 Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Answer :
It is given that pH of the solution is 10,i.e., the hydrogen ion concentration in the solution is 10 -10 M.
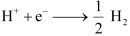
By Nernst equation we have :-
So,
or
So the required potential is - 0.591 V.
Question 3.5 Calculate the emf of the cell in which the following reaction takes place:
Given that
Answer :
Here we can directly apply the nernst equation :-
Putting the value in this equation :-
or
or
Hence the required potential is 0.914 V.
Answer :
For finding Gibbs free energy we know the relation :-
Now, for equilibrium constant we will use :-
So,
or
or
Question 3.7 Why does the conductivity of a solution decrease with dilution?
Answer :
The conductivity of a solution depends upon the number of ions and the distance between them. In the process of dilution, we don't increase the number of ions in the solution instead we increase the distance between them. So the conductivity of the solution decreases due to dilution.
Question 3.8 Suggest a way to determine the value of water.
Answer :
We know :
If we draw a straight line between and
, its slope will be -A and the intercept on the y-axis will be
.
In this way, we can obtain the value of limiting molar conductivity.
Question 3.8 The molar conductivity of methanoic acid is
Calculate its degree of dissociation and dissociation constant. Given and
Answer :
We know that :-
For degree of dissociation, we have :-
or
For dissociation constant, we have :-
or
or
Question 3.10 If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire?
Answer :
Firstly we will find total charge flown through the wire then we will calculate number of electrons.
We are given :- I = 0.5 A, Time = 2 hours = 7200 seconds.
We have, Q = I.t
= (0.5)7200 = 3600 C.
Now we will convert charge into number of electrons.
We know that
So toal number of electrons :
or no. of electrons will flow through wire.
Question 3.11 Suggest a list of metals that are extracted electrolytically.
Answer :
Metals like Na, Mg, Al, etc. are produced on a large scale by electrochemical reduction of their respective cations or by the process electrolysis because there are no suitable reducing agents available for this purpose.
Question 3.12 Consider the reaction:
What is the quantity of electricity in coulombs needed to reduce 1 mol of ?
Answer :
It is clear from the given reaction that reduction of 1 mol of Cr 2 O 7 2- will be
= 6 F (as 6 electrons are required to balance the reaction; Charge required = nF)
Thus 578922 C charge is required for reduction of 1 mol of Cr 2 O 7 2- .
Answer :
The lead storage battery can be recharged by reversing the direction of current passing through it.
For recharging PbSO 4 is converted into Pb at the anode and into PbO 2 at the cathode.
The chemical reactions are as follows:-

Question 3.14 Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
Answer :
The two materials are methane and methanol that can be used as fuels in fuel cells.
Question 3.15 Explain how rusting of iron is envisaged as setting up of a electrochemical cell.
Answer :
The chemistry of corrosion is quite complex but it can be understood by considering it as an electrochemical phenomenon. Consider a particular spot on an object where corrosion takes place. At here oxidation takes place and this spot behaves as the anode. The released electrons at anodic spot go through the metal and go to another spot on the metal and reduction of oxygen takes place in the presence of H+. This spot behaves as a cathode with the reaction. In this way this analogy is possible.
Answer :
The order in which metals displace each other from the solution of their salts can be given with the help of their standard electrode potential. Since magnesium has the least standard electrode potential so it is the most strong reducing agent. So the required order we get is:-
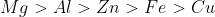
Question 3.2 Given the standard electrode potentials,
Arrange these metals in their increasing order of reducing power.
Answer :
Elements with reducing power or reducing agents have least/minimum standard electrode potential i.e., reducing power increases with a decrease in standard electrode potential. So the result obtained is:-
K > Mg > Cr > Hg > Ag
Question 3.3(i) Depict the galvanic cell in which the reaction
takes place.Further show
(i) Which of the electrode is negatively charged?
Answer :
The galvanic cell of the given reaction is depicted below :-
Zn (s) | Zn +2 (aq) || Ag + (aq) | Ag (s)
Clearly Zn electrode is negatively charged.
Question 3.3(ii) Depict the galvanic cell in which the reaction
takes place.
(ii) Further show: The carriers of the current in the cell.
Answer :
The carriers of current in the cell are ions . and Current flows from silver to zinc in the external circuit.
Question 3.3(iii) Depict the galvanic cell in which the reaction
takes place.
(iii) Further show: Individual reaction at each electrode.
Answer :
The reaction taking place at both cathode and anode are shown below :-
(i) Cathode reaction :-
(ii) Anode reaction :-
Question 3.4(i) Calculate the standard cell potentials of galvanic cell in which the following reactions take place:
Calculate the and equilibrium constant of the reactions.
Answer :
The galvanic cell of the given reaction is shown below:-
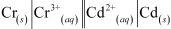
The standard electrode potential of Cr and Cd can be found in table of standard electrode potential.
So, we get :
Now
Putting values :
Now for finding equlilibrium constant we have :
or
or
Question 3.4(ii) Calculate the standard cell potentials of galvanic cell in which the following reactions take place:
Calculate the and equilibrium constant of the reactions.
Answer :
The galvanic cell of the given reaction is shown below :-
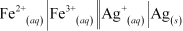
We can know about the electrode potential of Fe and Ag with the help of table of standard electrode potential.
We have :
or
or
Now consider :
or
Now for equilibrium constant :
or
or
Thus
Question 3.5(i) Write the Nernst equation and emf of the following cells at 298 K:
(i)
Answer :
The nernst equation gives :
This gives,
or
or
So the emf of the cell is 2.67 V.
Question 3.5(ii) Write the Nernst equation and emf of the following cells at 298 K:
(ii)
Answer :
The nernst equation for this gives :
This gives :
or
Thus the emf of the given galvanic cell is 0.53 V.
Question 3.5(iii) Write the Nernst equation and emf of the following cells at 298 K:
(iii)
Answer :
The nernst equation for this reaction gives :-
Now for emf, just put all the values.
or
or
Thus emf of the cell is 0.078 V.
Question 3.5(iv) Write the Nernst equation and emf of the following cells at 298 K:
Answer :
The nernst equation of the given reaction gives :
or
or
or
So the required emf of the cell is -1.298 V.
Question 3.6 In the button cells widely used in watches and other devices the following reaction takes place:
Determine and
for the reaction.
Answer :
The given reaction is obtained from :-
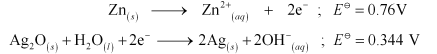
So the E o cell can be obtained directly.
Now for free energy calculation, we have :-
or
or
or
Question 3.7 Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
Answer :
Conductivity(k) or specific conductance of a solution is defined as the inverse of resistivity.
Mathematically, it can be written as:-
In the above equation is the conductivity of a solution. Thus the definition of conductivity becomes as the conductance of a substance which is 1 cm long and has 1 sq. cm of cross-sectional area.
With dilution conductivity of a solution decreases due to an increase in distance between ions.\
Molar conductivity: - It is defined as the conductivity of a solution per unit concentration
i.e.,
It is clear from the above mathematical expression of the molar conductivity that, if we dilute the solution or decrease its concentration then molar conductivity increases. This is because, on dilution of a solution, a decrease in is more than compensated by the increase in its volume.
Question 3.8 The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 S cm–1. Calculate its molar conductivity.
Answer :
We know that the molar conductivity of a solution is defined as:-
Putting the value of conductivity and concentration in the above equation:-
Answer:
We are given with conductivity of cell
and resistance R = 1500
.
Also, Cell constant =
or =
or =
Question 3.11 Conductivity of 0.00241 M acetic acid is . Calculate its molar conductivity. If
for acetic acid is
, what is its dissociation constant?
Answer :
Molar conductivity of a solution is given by :-
So,
or
Also, it is given that .
or
For dissociation constant we have,
so,
or
Question 3.12(i) How much charge is required for the following reductions:
(i)
Answer :
The equation becomes :-
So required charge is 3F.
Q = n*96500
Q = 3*96500 = 289500 C
Question 3.12(ii) How much charge is required for the following reductions:
(ii)
Answer :
The equation can be written as:-
Thus charge required is
Question 3.12(iii) How much charge is required for the following reductions:
(iii)
Answer :
The given reaction can be written as:-
Thus charge required in above equation
Question 3.13(i) How much electricity in terms of Faraday is required to produce
(i) 20.0 g of Ca from molten ?
Answer :
The equation for the question is given by :-
In this equation, for 1 mol of Ca, 2F charge is required or we can say that for 40 g of Ca charge required is 2F.
So, for 20 g of Ca charge required will be = F = 96500 C.
Question 3.13(ii) How much electricity in terms of Faraday is required to produce
(ii)40.0 g of AI from molten ?
Answer :
The equation for the given question is :-
Thus for 1 mol of Al, charge required is 3F.
So the required amount of electricity in terms of charge will be :-
Question 3.14(i) How much electricity is required in coulomb for the oxidation of
(i) 1 mol of ?
Answer :
According to question the equation of oxidation will be :-
Thus, for oxidation of O 2- , 2F charge is required.
Question 3.14(ii) How much electricity is required in coulomb for the oxidation of
(ii)1 mol of ?
Answer :
The oxidation equation for the given reaction will be :-
So for oxidation of 1 mol charge required
Answer :
We are given:
I = 5A
and t = 20(60) = 1200 sec.
So total charge = 5(1200) = 6000 C.
The equation for nickel deposition will be:-
Thus, from 2F charge 58.7 g of nickel deposition takes place.
i.e.,
So for 6000 C charge total nickel deposition will be:-
or
Hence 1.825 g Ni will be deposited in the given conditions.
Answer :
Since the cells are connected in series so the current passing through each cell will be equal.(1.5 A)
Now we are given that 1.45 g of silver is deposited. So firstly we will consider the cell containing silver.
Since for deposition of 108 g silver 96487 C charge is required, thus for 1.45 g deposition of silver charge required will be:-
Now we can find the time taken by 1.5 A current to deposit 1.45 g silver.
For copper:-
Since 2F charge will deposit 63.5 g of Cu, then deposition by 1295.43 C will be:-
Hence 0.426 g of copper will be deposited.
For zinc:-
Since 2F charge will deposit 65.4 g of Zn, then deposition by 1295.43 C will be:-
Hence 0.439 g of zinc will be deposited.
Question 3.17(i) Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(i)
Answer :
The concept used here will be that a reaction is feasible only if is positive.
Anode and cathode reactions will be as follows:-
So
So this reaction is feasible.
Question 3.17(ii) Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(ii)
Answer :
A reaction is feasible only if is positive.
So, anode and cathode reactions will be as follows :-
and
So this reaction is feasible.
Question 3.17(iii) Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(iii)
Answer :
A reaction is feasible only if is positive.
So, anode and cathode reactions will be as follows :-
and
So this reaction is not feasible.
Question 3.17(iv) Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
Answer :
A reaction is feasible only if is positive.
So, anode and cathode reactions will be as follows:-
and
So this reaction is not feasible.
Question 3.17(v) Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(v) 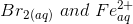 Answer :
Answer :
A reaction is feasible only if is positive.
So, anode and cathode reactions will be as follows :-
and
So this reaction is feasible.
Question 3.18(i) Predict the products of electrolysis in each of the following:
(i) An aqueous solution of with silver electrodes.
Answer :
For the given solution :
At cathode :- Reaction with greater E 0 will take place.
At anode :-
Hence, silver will get deposited at the cathode and it will be getting dissolved at anode.
Question 3.18(ii) Predict the products of electrolysis in each of the following:
(ii)An aqueous solution of with platinum electrodes.
Answer :
For the given solution :
At cathode :- Reaction with greater E 0 will take place.
At anode :- Self ionisation will take place due to presence of water.
Hence, silver will get deposited at the cathode and O 2 will be produced from anode.
Question 3.18(iii) Predict the products of electrolysis in each of the following:
(iii) A dilute solution of with platinum electrodes.
Answer :
For the given solution :
At cathode :- Reaction with greater E 0 will take place.
At anode :- Self ionisation of water will take place due to presence of platinum electrode.
Hence, H 2 gas will be generated at cathode and O 2 will be produced from anode.
Question 3.18(iv) Predict the products of electrolysis in each of the following:
(iv) An aqueous solution of with platinum electrodes.
Answer :
For the given solution :
At cathode :- Reaction with greater E 0 will take place.
At anode :-
Hence, Cu will get deposited at cathode and Cl 2 will be produced from anode.
Class 12 Chemistry Electrochemistry NCERT Solutions
This chapter of Class 12 NCERT solutions is the third chapter of NCERT Class 12 Chemistry book. Electrochemistry basically deals with concepts related to an electrochemical cell, electrolytic cell, Nernst equation, emf of a cell, Faraday's law, Battery, fuel cell, corrosion etc. NCERT Solutions for Class 12 Chemistry Chapter 3 does not have many linkages with the previous chapter however it deals with some terms mentioned in chapter 2 like concentration, molarity etc.
Class 12 NCERT solutions is for chapter Electrochemistry is a good source to cover it in a comprehensive manner. Students can score decent marks in this chapter as questions are mostly based on Faraday's law and if the concept is clear, questions can be handled easily. Apart from NCERT, students can refer to class notes for Chemistry Class 12 Chapter 3 to revise and score well in the final board examination as well as competitive exams.
Class 12 NCERT solutions provide questions that cover almost all the topics of the chapter. Hence it becomes inevitable to at least solve them once before the final examination. Class 12 Chemistry Chapter 3 Electrochemistry is a must to read for students as it has decent weightage in all the exams ranging from Board to NEET and JEE.
Ch 3 Chemistry Class 12 solutions will take 10-12 hours to complete if all the concepts of 11th class related to concentration of solutions, Anode, cathode, oxidation and reduction are well-read. Students can easily score more than 90 per cent marks in this chapter as limited concepts are there and the difficulty level of questions is not that high.
NCERT Exemplar Class 12 Solutions
Features of Electrochemistry Class 12 Solutions
Electrochemistry is an important chapter for both CBSE Board exam as well as competitive exams like JEE, NEET, BITSAT, VITEE and KVPY, etc. It carries 5 marks in the CBSE board exams hence learning the concepts of this chapter is very important to get good marks. In this chapter, there are 18 exercise questions. The step-by-step NCERT solutions for class 12 chemistry chapter 3 Electrochemistry are prepared by subject experts which not only help you to clear your doubts but also help you to improve your writing skills.
The NCERT solutions provided here are completely free of cost and you can also download them for offline usage. Please scroll down to get NCERT solutions for class 12 chemistry chapter 3 electrochemistry. By referring to the NCERT solutions for class 12 , students can understand all the important concepts and practice questions well enough before their examination.
NCERT Solutions for Class 12 Chemistry
Chapter 1 | |
Chapter 2 | |
Chapter 3 | Electrochemistry |
Chapter 4 | Chemical Kinetics |
Chapter 5 | |
Chapter 6 | |
Chapter 7 | |
Chapter 8 | |
Chapter 9 | |
Chapter 10 | |
Chapter 11 | |
Chapter 12 | |
Chapter 13 | |
Chapter 14 | |
Chapter 15 | |
Chapter 16 |
NCERT Solutions for Class 12 Subject wise
Benefits of CBSE NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry
Hope you have understood well with the help of the free solutions provided here. After completing the solutions of NCERT class 12 chemistry chapter 3 Electrochemistry, students will be able to describe an electrochemical cell, to differentiate between electrolytic and galvanic cells, to apply Nernst equation for calculating the emf of galvanic cell, also will be able to derive relation between standard potential of the cell, Gibbs energy of cell reaction and its equilibrium constant, etc. Keep working, keep improving and also keep enjoying.
Also Check NCERT Books and NCERT Syllabus here:
Frequently Asked Question (FAQs)
This chapter holds weightage of 2% for NEET exam. The weightage given is average based on the previous year papers. To practice questions on the chapter refer to NCERT exemplar problems and NCERT book exercises.
4 marks, that is 1 question can be expected from Electrochemistry for JEE Main exam. Follow previous year papers and NCERT books for good score in the exam.
Around 6 marks questions are asked from the NCERT syllabus of Class 12 chapter 3 Electrochemistry.
For complete solutions : https://school.careers360.com/ncert/ncert-solutions-class-12-chemistry Chapter wise links are provide to get NCERT solutions for Class 12 Chemistry PDF.
- Type of cells
- Electrode potential.
- Standard electrode potential.
- Anode.
- Cathode.
- Cell potential.
- Cell electromotive force (emf)
- SHE (Standard Hydrogen Electrode)
Also Read
Articles
Certifications By Top Providers
Explore Top Universities Across Globe
Questions related to CBSE Class 12th
Hello aspirant,
The purpose of graphic design extends beyond the brand's look. Nevertheless, by conveying what the brand stands for, it significantly aids in the development of a sense of understanding between a company and its audience. The future in the field of graphic designing is very promising.
There are various courses available for graphic designing. To know more information about these courses and much more details, you can visit our website by clicking on the link given below.
https://www.careers360.com/courses/graphic-designing-course
Thank you
Hope this information helps you.
hello,
Yes you can appear for the compartment paper again since CBSE gives three chances to a candidate to clear his/her exams so you still have two more attempts. However, you can appear for your improvement paper for all subjects but you cannot appear for the ones in which you have failed.
I hope this was helpful!
Good Luck
Hello dear,
If you was not able to clear 1st compartment and now you giving second compartment so YES, you can go for your improvement exam next year but if a student receives an improvement, they are given the opportunity to retake the boards as a private candidate the following year, but there are some requirements. First, the student must pass all of their subjects; if they received a compartment in any subject, they must then pass the compartment exam before being eligible for the improvement.
As you can registered yourself as private candidate for giving your improvement exam of 12 standard CBSE(Central Board of Secondary Education).For that you have to wait for a whole year which is bit difficult for you.
Positive side of waiting for whole year is you have a whole year to preparing yourself for your examination. You have no distraction or something which may causes your failure in the exams. In whole year you have to stay focused on your 12 standard examination for doing well in it. By this you get a highest marks as a comparison of others.
Believe in Yourself! You can make anything happen
All the very best.
Hello Student,
I appreciate your Interest in education. See the improvement is not restricted to one subject or multiple subjects and we cannot say if improvement in one subject in one year leads to improvement in more subjects in coming year.
You just need to have a revision of all subjects what you have completed in the school. have a revision and practice of subjects and concepts helps you better.
All the best.
Hi,
You just need to give the exams for the concerned two subjects in which you have got RT. There is no need to give exam for all of your subjects, you can just fill the form for the two subjects only.
Popular CBSE Class 12th Questions
Colleges After 12th
Also Read
Applications for Admissions are open.

JEE Main Important Physics formulas
ApplyAs per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

UPES School of Liberal Studies
ApplyRanked #52 Among Universities in India by NIRF | Up to 30% Merit-based Scholarships | Lifetime placement assistance

Aakash iACST Scholarship Test 2024
ApplyGet up to 90% scholarship on NEET, JEE & Foundation courses

JEE Main Important Chemistry formulas
ApplyAs per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

PACE IIT & Medical, Financial District, Hyd
ApplyEnrol in PACE IIT & Medical, Financial District, Hyd for JEE/NEET preparation

ALLEN JEE Exam Prep
ApplyStart your JEE preparation with ALLEN
