Apply to Aakash iACST Scholarship Test 2024
NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions
NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions- In NCERT Class 12 Chemistry chapter 2 Solutions, there are direct answers to the 41 questions which are there in the chapter exercise. The students will be able to find step-by-step NCERT solutions for Class 12 Chemistry Chapter 2 Solutions which will eventually help you to write good answers and get good marks in the CBSE exam. The NCERT solutions which are provided here are free of cost and are easily accessible. By referring to the NCERT solutions for class 12, students can understand all the important concepts and practice questions well.
Latest: JEE Main high scoring chapters
Don't Miss: JEE Main 10 year's papers
Recommended: NEET high scoring chapters
Suggested: NEET question papers with solutions
In our daily life, we come across various mixtures like soft drinks, syrups, and air. All of them are mixtures of two or more pure substances like air is a mixture of mainly nitrogen and oxygen etc. Also, you know about various types of mixtures or solutions like gaseous solutions, liquid solutions, and solid solutions. These topics of NCERT solutions for Class 12 Chemistry Chapter 2 Solutions are not only important for the CBSE 12th exam but also for the various competitive exams like JEE Mains, NEET, BITSAT, VITEEE, etc.
Also Read,
NCERT Exemplar Solutions Class 12 Chemistry Chapter 2
NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions
NCERT chemistry class 12 intext questions solutions chapter 2: Exercise 2.1 to 2.12
Answer :
We know that solute and solvent forms solution.
So mass percentage of benzene (solute) :-
Similarly mass percentage of CCl 4 :-
Question 2.2 Calculate the mole fraction of benzene in solution containing by mass in carbon tetrachloride.
Answer :
For calculating mole fraction, we need moles of both the compounds.
It is given that benzene is in the solution by mass.
So if we consider 100g of solution then 30g is benzene and 70g is CCl 4 .
Similarly moles of benzene :
So mole fraction of benzene is given :
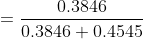
Question 2.3(a) Calculate the molarity of each of the following solutions:
of
in
of solution
Answer :
For finding molarity we need the moles of solute and volume of solution.
So moles of solute :
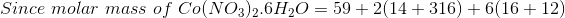
Now,
Question 2.3(b) Calculate the molarity of each of the following solutions:
of
diluted to
.
Answer :
By conservation of moles we can write :
M 1 V 1 = M 2 V 2
Given that M 1 = 0.5 M and V 1 = 30 ml ; V 2 = 500 ml
Question 2.4 Calculate the mass of urea required in making
of
aqueous solution.
Answer :
Let us assume that the mass of urea required be x g.
So moles of urea will be :
we get x = 37
Thus mass of urea required = 37 g.
Question 2.5 Calculate
(a) molality
(b) molarity and
(c) mole fraction
of KI if the density of (mass/mass) aqueous KI is
.
Answer :
If we assume our solution is 100 g. Then according to question, 20 g KI is present and 80 g is water.
So moles of KI :
(a) Molality :-
(b) Molarity :-
(c) Mol fraction :- Moles of water :-
So, mol fraction of KI :-
Answer :
For finding Henry's constant we need to know about the mole fraction of H 2 S.
Solubility of H 2 S in water is given to be 0.195 m .
i.e., 0.195 moles in 1 Kg of water.
So
At STP conditions, pressure = 1 atm or 0.987 bar
Equation is :
So we get :
Question 2.7 Henry’s law constant for in water is
at
Calculate the quantity of
in
of soda water when packed under
pressure at
Answer :
We know that ,
Pressure of CO 2 = 2.5 atm
We know that :
So, Pressure of CO 2 = Pa
By Henry Law we get,
Taking density of soda water = 1 g/ml
We get mass of water = 500 g.
So, Moles of water :
Also,
So, moles of CO 2 = 0.042 mol
Using relation of mole and given mass, we get
Mass of CO 2 = 1.848 g.
Answer :
Let the composition of liquid A (mole fraction) be x A .
So mole fraction of B will be x B = 1 - x A .
Given that,
Using Raoult’s law ,
Putting values of p total and vapour pressure of pure liquids in the above equation, we get :
600 = 450.x A + 700.(1 - x A )
or 600 - 700 = 450x A - 700x A
or x A = 0.4
and x B = 0.6
Now pressure in vapour phase :
= 450(0.4) = 180 mm of Hg
= 700(0.6) = 420 mm of Hg
And mole fraction of liquid B = 0.70
Question 2.9 Vapour pressure of pure water at is
of urea
is dissolved in
of water. Calculate the vapour pressure of water for this solution and its relative lowering.
Answer :
Given that vapour pressure of pure water,
Moles of water :
Moles of urea :
Let the vapour pressure of water be p w .
By Raoult's law, we get :
or
or p w = 23.4 mm of Hg.
Relative lowering :- Hence, the vapour pressure(v.P) of water in the solution = 23.4 mm of Hg
and its relative lowering = 0.0173.
Question 2.10 Boiling point of water at is
. How much sucrose is to be added to
of water such that it boils at
Answer :
Here we will use the formula :
Elevation in temperature = 100 - 99.63 = 0.37
K b = 0.52 ;
Putting all values in above formula, we get :
Thus 121.67 g of sucrose needs to be added.
Question 2.11 Calculate the mass of ascorbic acid (Vitamin C, ) to be dissolved in
of acetic acid to lower its melting point by
.
Answer :
Elevation in melting point = 1.5 degree celsius.
Here we will use the following equation :
Putting given values in the above equation :
Thus 5.08 ascorbic acid is needed for required condition.
Question 2.12 Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving of polymer of molar mass
in
of water at
Answer :
We know that :
We are given with :-
Volume, V = 0.45 L
Thus osmotic pressure :
NCERT solutions for class 12 chemistry chapter 2 Solutions : Exercises
Solution :- A solution is a homogeneous mixture of two or more non-reacting substances. It has two components :- solute and solvent.
Types of solutions are given below :-
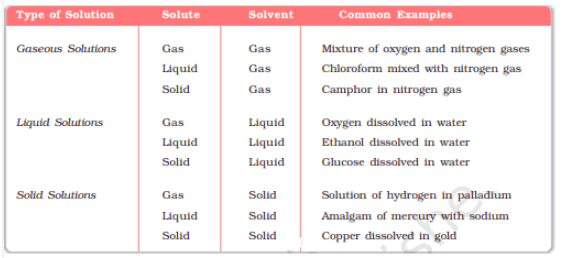
![]()
Q.2.2 Give an example of a solid solution in which the solute is a gas.
Answer:
Solution of hydrogen in palladium is such an example in which solute is a gas and solvent is solid.
Q.2.3(i) Define the following terms:
Mole fraction
Answer :
Mole fraction is defined as the ratio of number of moles of a component and total number of moles in all components.
i.e.,
Q.2.3(ii) Define the following terms:
Molality
Answer :
It is defined as the number of moles of solute dissolved per kg (1000g) of solvent
i.e.,
It is independent of temperature.
Q2.3(iii) Define the following terms:
Molarity
Answer :
Molarity is defined as number of moles of solute dissolved per litre(or 1000ml) of solution.
i.e.,
It depends on temperature because volume is dependent on temperature.
Q2.3(iv) Define the following terms:
Mass percentage.
Answer :
Mass percentage is defined as the percentage ratio of mass of one component to the total mass of all the components.
i.e.,
Answer :
According to given question, in 100 g of solution 68 g is nitric acid and rest is water.
So moles of 68 g HNO 3 :-
Density of solution is given to be 1.504.
So volume of 100 g solution becomes :-
Thus, molarity of nitric acid is :
Answer :
According to question, mass percentage means in 100 g of solution 10 g glucose is dissolved in 90 g water.
Molar mass of glucose (C 6 H 12 O 6 ) =
So moles of glucose are :
Mole fraction :-
Molarity :- Volume of 100 g solution :
Answer :
Total amount of mixture of Na 2 CO 3 and NaHCO 3 = 1 g.
Let the amount of Na 2 CO 3 be x g.
So the amount of NaHCO 3 will be equal to (1 - x) g.
Now it is given that it is an equimolar mixture.
So, Moles of Na 2 CO 3 = Moles of NaHCO 3 .
or
or x = 0.558 g
So
and
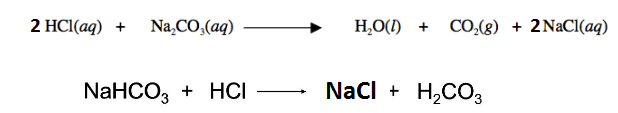
It is clear that for 1 mol of Na 2 CO 3 2 mol of HCl is required, similarly for 1 mol of NaHCO 3 1 mol of HCl is required.
So number of moles required of HCl = 2(0.00526) + 0.0053 = 0.01578 mol
It is given that molarity of HCl is 0.1 which means 0.1 mol of HCl in 1l of solution.
Thus required volume :
Answer :
According to question we have 2 solute,
Solute 1. : of 300 g gives :
Solute 2. : of 400 g gives :
So total amount of solute = 75 + 160 = 235 g.
Thus mass percentage of solute is :
and mass percentage of water
Answer :
For finding molality we need to find the moles of ethylene glycol.
Moles of ethylene glycol :
We know that :
Now for molarity :-
Total mass of solution = 200 + 222.6 = 422.6 g
Volume of solution
So molarity :-
express this in percent by mass
Answer :
We know that 15 ppm means 15 parts per million.
Required percent by mass :
determine the molality of chloroform in the water sample.
Answer :
Moles of chloroform :
Mass of water is . (Since contamination is 15 ppm)
So molality will be :
Q2.10 What role does the molecular interaction play in a solution of alcohol and water?
Answer :
Both alcohol and water individually have strong hydrogen bonds as their force of attraction. When we mix alcohol with water they form solution due to the formation of hydrogen bonds but they are weaker as compared to hydrogen bonds of pure water or pure alcohol.
Thus this solution shows a positive deviation from the ideal behaviour.
Q2.11 Why do gases always tend to be less soluble in liquids as the temperature is raised?
Answer:
It is known that dissolution of gas in a liquid is an exothermic process. So, by Le Chatelier principle we know that equilibrium shifts backwards as we increase temperature in case of exothermic process. Thus gases always tend to be less soluble in liquids as the temperature is raised.
Q2.12 State Henry’s law and mention some important applications.
Answer :
According to Henry's law at a constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of solution or liquid.
i.e., p = k h x, Here k h is Henry’s law constant.
Some of its applications are as follows:-
(a) We can increase the solubility of CO 2 in soft drinks, the bottle is sealed under high pressure.
(b) To avoid bends (due to blockage of capillaries) and the toxic effects of high concentrations of nitrogen in the blood, the tanks used by scuba divers are filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen).
(c) The partial pressure of oxygen is less at high altitudes than that at the ground level. This leads to low concentrations of oxygen in the blood and tissues climbers. Due to low blood oxygen, climbers become weak and unable to think clearly which are symptoms of a condition known as anoxia.
Answer :
Using Henry's Law we can write,
Putting value in this equation, we get :
So, the magnitude of k is .
Now, we will again use the above equation for .
So the required partial pressure is :-
or
Answer :
Positive and negative deviation: - A non-ideal solution is defined as a solution which does not obey Raoult’s law over the entire range of concentration i.e., and
. The vapour pressure of these solutions is either higher or lower than that expected by Raoult’s law. If vapour pressure is higher, the solution shows a positive deviation and if it is lower, it shows a negative deviation from Raoult’s law.
Enthalpy relation to positive and negative deviation can be understood from the following example:-
Consider a solution made up of two components - A and B. In the pure state the intermolecular force of attraction between them are A-A and B-B. But when we mix the two, we get a binary solution with molecular interaction A-B.
If A-B interaction is weak than A-A and B-B then enthalpy of reaction will be positive thus reaction will tend to move in a backward direction. Hence molecules in binary solution will have a higher tendency to escape. Thus vapour pressure increases and shows positive deviation from the ideal behaviour.
Similarly, for negative deviation, A-B interaction is stronger than that of A-A and B-B.
Answer :
It is given that of aq. solution. This means 2 g of non-volatile solute in 98 g of H 2 O.
Also the vapour of water at normal boiling point = 1.013 bar.
Using Raoult's law :
So we get :
Thus the molar mass of non-volatile solute is 41.35 unit.
Answer :
Vapour pressure of heptane =
and vapour pressure of octane =
Firstly we will find moles of heptane and octane so that we can find vapour pressure of each.
Molar mass of heptane = 7(12) + 16(1) = 100 unit.
and molar mass of octane = 8(12) + 18(1) = 114 unit.
So moles of heptane :
and moles of octane :
Mole fraction of heptane = 0.456 and mole fraction of octane = 0.544
Now we will find the partial vapour pressure:-
(i) of heptane :-
(ii) of octane :-
So total pressure of solution =
= 47.97 + 25.46 = 73.43 KPa
Answer :
It is asked the vapour pressure of 1 molal solution which means 1 mol of solute in 1000 g H 2 O.
Moles in 1000g of water = 55.55 mol. (Since the molecular weight of H 2 O is 18)
Mole fraction of solute :
Applying the equation :
or
or
Thus the vapour pressure of the solution is 12.083 KPa
Answer :
Let the initial vapour pressure of octane = .
After adding solute to octane, the vapour pressure becomes :
Moles of octane :
Using Raoult's law we get :
or
or
Thus required mass of non-volatile solute = 10g.
molar mass of the solute
Answer :
In this question we will find molar mass of solute by using Raoult's law .
Let the molar mass of solute is M.
Initially we have 30 g solute and 90 g water.
Moles of water :
By Raoult's law we have :-
or
or ------------------------------ (i)
Now we have added 18 g of water more, so the equation becomes:
Moles of H 2 O :
Putting this in above equation we obtain :-
or -----------------------------------(ii)
From equation (i) and (ii) we get
M = 23 u
So the molar mass of solute is 23 units.
vapour pressure of water at 298 K.
Answer :
In the previous part we have calculated the value of molar mass the Raoul's law equation.
We had :-
Putting M = 23 u in the above equation we get,
or
Thus vapour pressure of water = 3.53 kPa.
Answer :
It is given that freezing point of pure water is 273.15 K.
So, elevation of freezing point = 273.15 - 271 = 2.15 K
solution means 5 g solute in 95 g of water.
Moles of cane sugar :
Molality :
We also know that -
or
Now we will use the above procedure for glucose.
of glucose means 5 g of gluocse in 95 g of H 2 O.
Moles of glucose :
Thus molality :
So, we can find the elevation in freezing point:
Thus freezing point of glucose solution is 273.15 - 4.09 = 269.06 K.
Answer :
In this question we will use the formula :
Firstly for compound AB 2 :-
or
Similarly for compound AB 4 :-
If we assume atomic weight of element A to be x and of element B to be y, then we have :-
x + 2y = 110.87 ----------------- (i)
x + 4y = 196 ----------------- (ii)
Solving both the equations, we get :-
x = 25.59 ; y = 42.6
Hence atomic mass of element A is 25.59u and atomic mass of element B is 42.6u.
Answer :
According to given conditions we have same solution under same temperature. So we can write :
So, if we put all the given values in above equation, we get
or
Hence the required concentration is 0.061 M.
Q2.23(i) Suggest the most important type of intermolecular attractive interaction in the following pairs.
n-hexane and n-octane
Answer :
Since both the compounds are alkanes so their mixture has van der Waal force of attraction between compounds.
Q2.23(ii) Suggest the most important type of intermolecular attractive interaction in the following pairs.
Answer :
The binary mixture of these compounds has van der Waal force of attraction between them.
Q2.23(iii) Suggest the most important type of intermolecular attractive interaction in the following pairs.
Answer :
The given compounds will have ion-dipole interaction between them.
Q2.23(iv) Suggest the most important type of intermolecular attractive interaction in the following pairs.
Answer :
Methanol has -OH group and acetone has ketone group. So there will be hydrogen bonding between them.
Q2.23(v) Suggest the most important type of intermolecular attractive interaction in the following pairs.
acetonitrile and acetone
Answer :
They will have dipole-dipole interaction since both are polar compounds.
Cyclohexane, KCl, CH3OH, CH3CN.
Answer :
The order will be : Cyclohexane > CH 3 CN > CH 3 OH > KCl
In this, we have used the fact that like dissolves like.
Since cyclohexane is an alkane so its solubility will be maximum.
phenol
Answer :
We know the fact that like dissolves in like.
Since phenol is had both polar and non-polar group so it is partially soluble in water.
toluene
Answer :
Since toluene is a non-polar compound i.e., it doesn't have any polar group so it is insoluble in water. (because water is a polar compound)
formic acid
Answer :
Since the -OH group in formic acid (polar) can form H-bonds with water thus it is highly soluble in water.
ethylene glycol
Answer :
Ethylene glycol is an organic compound but is polar in nature. Also, it can form H-bonds with water molecules, thus it is highly soluble in water.
chloroform
Answer :
Chloroform is a non-polar compound so it is insoluble in water.
pentanol
Answer :
Pentanol has both polar and non-polar groups so it is partially soluble in water.
Answer :
We know that, Molality :
So, for moles of solute we have :
Thus, molality :
Molality of Na + ions is 4m.
Q2.27 If the solubility product of is
, calculate the maximum molarity of
in aqueous solution.
Answer :
We are given,
The dissociation equation of CuS is given by :-
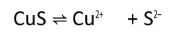
So, the equation becomes :-
or
or
Thus maximum molarity of solution is .
Q2.28 Calculate the mass percentage of aspirin in acetonitrile
when
of
is dissolved in
of
.
Answer :
Total mass of solution = Mass of aspirin + Mass of acetonitrile = 6.5 + 450 = 456.5 g.
We know that :
So,
Thus the mass percentage of aspirin is
Answer :
We are given with molality of the solution, so we need to find the moles of Nalorphene.
Molar mass of nalorphene = 19(12) + 21(1) + 1(14) + 3(16) = 311u.
So moles of nalorphene :
Molality :
or
or
So the required weight of water is 3.2 g.
Q2.30 Calculate the amount of benzoic acid required for preparing
of
solution in methanol.
Answer :
Molar mass of benzoic acid = 7(12) + 6(1) + 2(16) = 122u.
We are given with the molarity of solution.
or
or
So mass of benzoic acid :
Hence the required amount of benzoic acid is 4.575 g.
Answer :
We know that depression in freezing point of water will depend upon the degree of ionisation.
The degree of ionisation will be highest in the case of trifluoroacetic acid as it is most acidic among all three.
The order of degree of ionisation on the basis if acidic nature will be:- Trifluoroacetic acid > Trichloroacetic acid > Acetic acid.
So the depression in freezing point will be reverse of the above order.
Q2.32 Calculate the depression in the freezing point of water when of
is added to
of water.
Answer :
Firstly we will find the Vant's Hoff factor the dissociation of given compound.
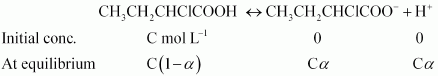
So we can write,
or
or
Putting values of K a and C in the last result, we get :
At equilibrium i = 1 - a + a + a = 1 + a = 1.0655
Now we need to find the moles of the given compound CH 3 CH 2 CHClCOOH.
So, moles =
Thus, molality of the solution :
Now we will use :
or
Answer :
Firstly we need to calculate molality in order to get vant's hoff factor.
So moles of CH 2 FCOOH :
We need to assume volume of solution to be nearly equal to 500 mL. (as 500 g water is present)
Now, we know that :
or
Now for dissociation constant :-
and,
Put values of C and a in the above equation, we get :
Answer :
Firstly we will find number of moles of both water and glucose.
Moles of glucose :
and moles of water :
Now,
or
or
Thus vapour pressure of water after glucose addition = 17.44 mm of Hg
Answer :
We know that :
We are given value of P and k, so C can be found.
Hence solubility of methane in benzene is .
Answer :
For calculating partial vapour pressure we need to calculate mole fractions of components.
So number of moles of liquid A :
and moles of liquid B :
Mole fraction of A (x A ) :
and mole fraction of B (x B ) :
Now, P total = P A + P B
or
or
or
Thus vapour pressure in solution due to A =
Plot this data also on the same graph paper. Indicate whether it has positive deviation or negative deviation from the ideal solution.
Answer :
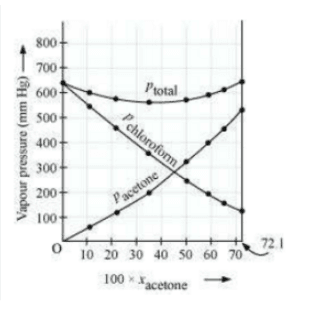
![]()
it has negative deviation from the ideal solution.
Answer :
Firstly, we will find the no. of moles of the given compounds.
No. of moles of benzene :
and the no. of moles of toluene :
.
Now we will find mol fraction of both:-
Mole fraction of benzene :-
and mole fraction of toluene :
Now,
P total = P b + P t
or
or
Hence mole fraction of benzene in vapour phase is given by :
Answer :
We have been given that the water is in equilibrium with air at a pressure of 10 atm or 7600 mm of Hg.
So the partial pressure of oxygen :
and partial pressure of nitrogen :
Now, by Henry's Law :
For oxygen :
For nitrogen :
Hence the mole fraction of nitrogen and oxygen in water is and
respectively.
Q2.41 Determine the amount of
dissolved in
of water such that its osmotic pressure is
at
.
Answer :
We know that osmotic pressure :
or
We have been given the values of osmotic pressure, V, i and T.
So the value of w can be found.
Hence 3.42 g CaCl 2 is required.
Answer :
Dissociation of K 2 SO 4 is as follows :-
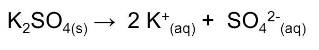 It is clear that 3 ions are produced, so the value of i will be 3.
It is clear that 3 ions are produced, so the value of i will be 3.
Molecular weight of K 2 SO 4 = 2(39) + 1(32) + 4(16) = 174u.
Putting all the values :-
More About Class 12 Chemistry Chapter 2 Solutions
NCERT Class 12 Chemistry chapter 2 solutions mainly discuss questions based on liquid solutions and their properties. The NCERT solutions for Class 12 Chemistry chapter 2 Solutions also cover other questions based on important concepts like types of solutions, Raoult's law and Henry's law, the concentration of solutions in different units, solubility, the vapour pressure of liquid solutions, ideal and non-ideal solutions, colligative properties, determination of molar mass and abnormal molar masses.
Topics and Sub-topics of NCERT Class 12 Chemistry Chapter 2 Solutions-
2.1Types of Solutions
2.2 Expressing Concentration of Solutions
2.3 Solubility
2.4 Vapour Pressure of Liquid Solutions
2.5 Ideal and Non-ideal Solutions
2.6 Colligative Properties and Determination of Molar Mass
2.7 Abnormal Molar Masses
This chapter of class 12 NCERT solutions is the second chapter of NCERT Class 12. It basically introduces basic concepts related to concentration, molarity, molality, mole fraction, Raoult's law, henry law, vapour pressure, colligative properties etc. NCERT solutions for Class 12 Chemistry Chapter 2 is an extension of chapter 1 class 11 NCERT also known as some basic concepts of chemistry. Class 12 NCERT solutions is very useful in the subsequent chapters like thermodynamics and Equilibrium etc. Ch 2 Chemistry Class 12 is very easy if basic concepts are understood well. Students can score decent marks in this chapter as most of the questions are form colligative properties and vapour pressure concepts which are easy to comprehend in the examination. Apart from NCERT, students can refer class notes for Chemistry Class 12 Chapter 2 to revise and score well in the final board examination as well as competitive exams.
Class 12 NCERT solutions has good amount of weightage in exams like NEET and JEE as well. Class 12 Chemistry Chapter 2 solutions is must read for the 12th class students. Most of the concepts like Molarity, molality, mole fraction, percentage composition etc. have been discussed in class 11 only, hence it is not very difficult to grasp the subsequent topics Henrys law, Colligative properties. Hence it is generally recommended to study class 11 chapters well before entering class 12th as heavy portion of syllabus is interlinked. Ch 2 Chemistry Class 12 solutions will take 8-10 hours to complete if all the concepts of 11th class are well read.
NCERT Exemplar Class 12 Solutions
NCERT solutions for class 12 chemistry
Chapter 1 | |
Chapter 2 | Solutions |
Chapter 3 | |
Chapter 4 | |
Chapter 5 | |
Chapter 6 | |
Chapter 7 | |
Chapter 8 | |
Chapter 9 | |
Chapter 10 | |
Chapter 11 | |
Chapter 12 | |
Chapter 13 | |
Chapter 14 | |
Chapter 15 | |
Chapter 16 |
SAT® | CollegeBoard
Registeration closing on 19th Apr for SAT® | One Test-Many Universities | 90% discount on registrations fee | Free Practice | Multiple Attempts | no penalty for guessing
TOEFL ® Registrations 2024
Thinking of Studying Abroad? Think the TOEFL® test. Save 10% on your TOEFL exam with ApplyShop gift cards!
NCERT solutions for class 12 subject wise
Benefits of NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions
- Hope you have understood well with the help of the free NCERT class 12 Chemistry chapter 2 solutions provided here.
- After completing NCERT solutions for class 12 chemistry chapter 2 Solutions, students will be able to differentiate between the types of solutions characteristics of ideal and non-ideal solutions, define solubility and colligative properties, understand abnormal molar mass.
- NCERT class 12 Chemistry chapter 2 pdf which you read here will also help you in building your concepts as well as a strong base in the subject. These will also help you in various competitive exams.
- With the help of these NCERT class 12 Chemistry chapter 2 solutions pdf download, you will be able to write answers well.
Also Check NCERT Books and NCERT Syllabus here:
- NCERT Books Class 12 Chemistry
- NCERT Syllabus Class 12 Chemistry
- NCERT Books Class 12
- NCERT Syllabus Class 12
Happy Learning!!
Frequently Asked Question (FAQs)
- Henry’s law
- Solubility of gases in liquids
- Colligative properties
- Raoult's law
- Relative lowering of vapour pressure
- Elevation of boiling point
- Osmotic pressure
- Abnormal molecular mass
- Van't Hoff factor
For complete solutions : https://school.careers360.com/ncert/ncert-solutions-class-12-chemistry
4 marks questions can be expected. Practice JEE Main previous year papers to understand the question type asked.
Weightage of solutions is 5% . For good score follow NCERT textbook and solve NEET previous year papers.
Also Read
Articles
Certifications By Top Providers
Explore Top Universities Across Globe
Questions related to CBSE Class 12th
Hello aspirant,
The purpose of graphic design extends beyond the brand's look. Nevertheless, by conveying what the brand stands for, it significantly aids in the development of a sense of understanding between a company and its audience. The future in the field of graphic designing is very promising.
There are various courses available for graphic designing. To know more information about these courses and much more details, you can visit our website by clicking on the link given below.
https://www.careers360.com/courses/graphic-designing-course
Thank you
Hope this information helps you.
hello,
Yes you can appear for the compartment paper again since CBSE gives three chances to a candidate to clear his/her exams so you still have two more attempts. However, you can appear for your improvement paper for all subjects but you cannot appear for the ones in which you have failed.
I hope this was helpful!
Good Luck
Hello dear,
If you was not able to clear 1st compartment and now you giving second compartment so YES, you can go for your improvement exam next year but if a student receives an improvement, they are given the opportunity to retake the boards as a private candidate the following year, but there are some requirements. First, the student must pass all of their subjects; if they received a compartment in any subject, they must then pass the compartment exam before being eligible for the improvement.
As you can registered yourself as private candidate for giving your improvement exam of 12 standard CBSE(Central Board of Secondary Education).For that you have to wait for a whole year which is bit difficult for you.
Positive side of waiting for whole year is you have a whole year to preparing yourself for your examination. You have no distraction or something which may causes your failure in the exams. In whole year you have to stay focused on your 12 standard examination for doing well in it. By this you get a highest marks as a comparison of others.
Believe in Yourself! You can make anything happen
All the very best.
Hello Student,
I appreciate your Interest in education. See the improvement is not restricted to one subject or multiple subjects and we cannot say if improvement in one subject in one year leads to improvement in more subjects in coming year.
You just need to have a revision of all subjects what you have completed in the school. have a revision and practice of subjects and concepts helps you better.
All the best.
Hi,
You just need to give the exams for the concerned two subjects in which you have got RT. There is no need to give exam for all of your subjects, you can just fill the form for the two subjects only.
Popular CBSE Class 12th Questions
Colleges After 12th
Also Read
Applications for Admissions are open.

JEE Main Important Physics formulas
ApplyAs per latest 2024 syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters

UPES School of Liberal Studies
ApplyRanked #52 Among Universities in India by NIRF | Up to 30% Merit-based Scholarships | Lifetime placement assistance

Aakash iACST Scholarship Test 2024
ApplyGet up to 90% scholarship on NEET, JEE & Foundation courses

JEE Main Important Chemistry formulas
ApplyAs per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

PACE IIT & Medical, Financial District, Hyd
ApplyEnrol in PACE IIT & Medical, Financial District, Hyd for JEE/NEET preparation

ALLEN JEE Exam Prep
ApplyStart your JEE preparation with ALLEN
