Browse by Stream
-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


- Home
- Engineering
- In the following reaction : <img alt="CH_{3}-OH + CH_{3}COCl \rightarrow P + H_{2}O \rightarrow Q + R" src="https://learn.careers360.com/latex-image/?CH_%7B3%7D-OH%20+%20CH_%7B3%
- #Telangana Engineering Agriculture and Medical Common Entrance Test
- #Amrita Engineering Entrance Examination
- #Chemistry
- #Engineering
- #Organic Compounds containing Oxygen
In the following reaction :
Q and R respectively are,
Option: 1
HCOOH + C2H5OH
Option: 2
CH3COOH + C2H5OH
Option: 3
HCOOH + CH3OH
Option: 4
CH3COOH + CH3OH
Answers (1)

As we learnt ,
Acidic and basic hydrolysis of Esters -
Acidic hydrolysis gives carboxylic acid directly. Basic hydrolysis gives carboxylates which acidify to give the carboxylic acid.
- wherein
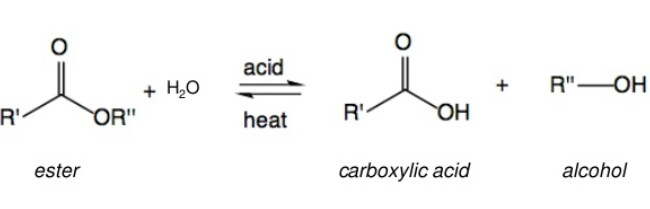

Therefore, Option(4) is correct
View full answer


