-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


- Home
- Engineering
- the chemical nature of hydrogen peroxide
The chemical nature of hydrogen peroxide is:
Option 1) Oxidising agent in acidic medium but not in basic medium.
Option 2) Reducing agent in basic medium , but not in acidic medium .
Option 3) Oxidising and reducing agent in acidic medium , but not in basic medium .
Option 4) Oxidising and reducing agent in both acidic and basic medium
Answers (1)
Hydrogen Peraoxide -
Discoverd by J - L Thena
- wherein
Structure of H2O2 (Hydrogen Peroxide) -
It is non planar molecule.
- wherein
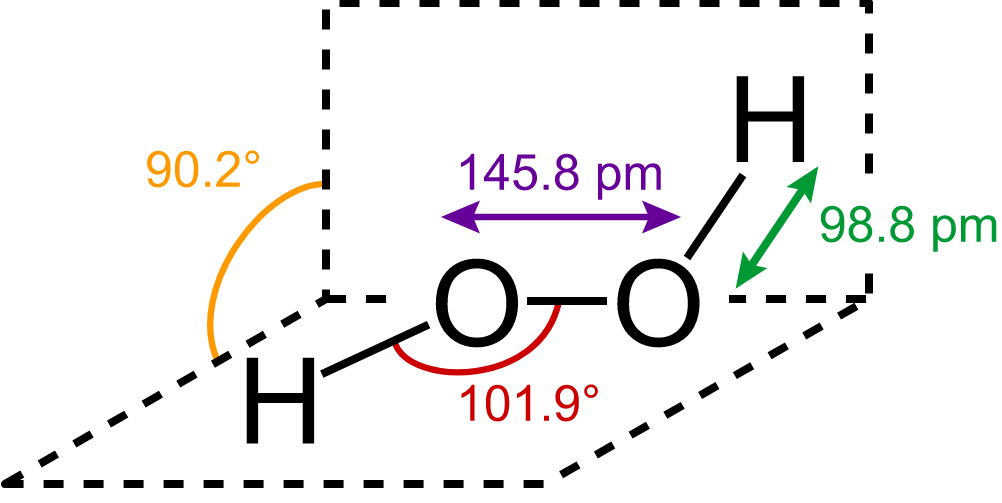
Decomposition -
It decomposes slowly on exposure to light
- wherein
Acidic Nature -
It is a weak acid turn blue litmus red only in pure state.
- wherein
Oxidising Nature -
It acts as oxidising agent in both acidic and alkaline solution.
- wherein
Acidic solution H2O2 reduced to H2O
Reducing Nature -
H2O2 acts as reducing agent.
- wherein
H2O2 get oxidised to O2
Bleaching Properties -
It bleaches delicates materials like Silk, Wool.
- wherein
Colouring matter +
Colourless
As we have learnt in peroxides and superoxides
H2O2 acts as oxidising agent and reducing agent in both acidic and basic medium.
As a oxidnat:
H2O2 acts as a reductant


