Browse by Stream
-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


- Home
- Engineering
- Which of the following is the wrong statement?Option: 1 Ozone is diamagnetic gasOption: 2 <
- #Engineering
- #Birla Institute of Technology & Science Admission Test
- #National Eligilibility Cum Entrance Test
- #Chemistry
- #p- Block Elements
Which of the following is the wrong statement?
Option: 1
Ozone is diamagnetic gas
Option: 2
and
are isoelectronic
Option: 3
molecule is bent.
Option: 4
Ozone is violet - black in solid state.
Answers (1)

Isoelectronic species are those which have the same number of electrons.
and
have different number of electrons.
= 8+7+17=32 electron
=8+7+8+1=24 electron
is a bent-shaped molecule. The structure of Ozone is given as
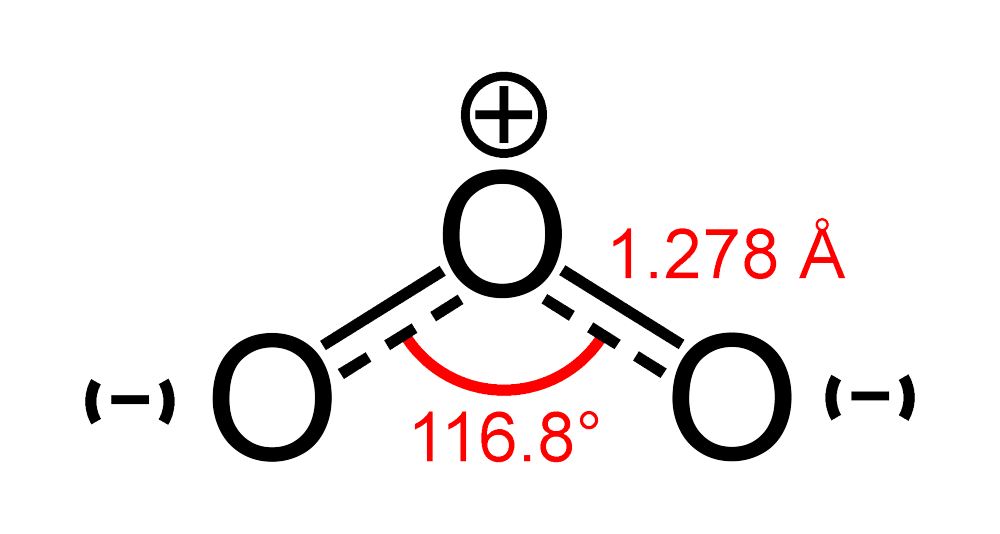
And is diamagnetic gas and it exists as a pale blue gas, dark blue liquid and violet-black solid
Therefore, option (2) is correct.
View full answer


