Browse by Stream
-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Exams
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


- Home
- Engineering
- Let respectively denotes the mean speed,root me
Let respectively denotes the mean speed,root mean square speed of the molecule in ideal monoatomic gas at absolute temperature T. The mass of the molecule is m then
Option: 1
No molecules can have speed greater than .
Option: 2
No molecules can have speed less than
Option: 3
Option: 4
The average kinetic charge of a molecule
Answers (1)
Most Probable Kinetic Energy -
The kinetic energy of maximum fraction of molecules, i.e., the peak in the fraction of molecules vs. kinetic energy graph is called most probable kinetic energy.
- wherein
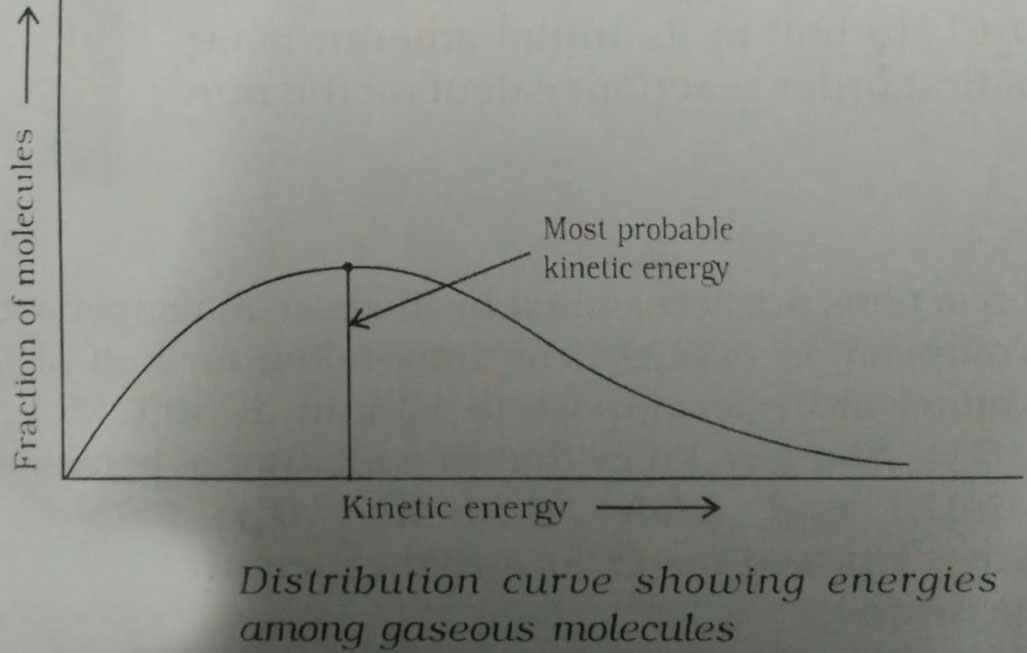
So
Avg.Kinetic Energy
View full answer

