-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


- Home
- Engineering
- Simplified adsorption spectrum of there complexed <img alt="\left (i) , (ii) and (iii)\right of , M^{nt} ion" src="https://learn.careers360.com/latex-image/?%5Cleft%20%28i%29%20%2C%20%28ii%29%20and%20%28iii%29%5Cright%20of%20%2C%20M%5E%7Bn
Simplified adsorption spectrum of there complexed 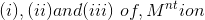 are provided below ; their
are provided below ; their 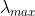 values are marked as
values are marked as 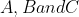 respectively. The correct match between the complexed and their
respectively. The correct match between the complexed and their 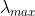 value is :
value is :

![\left [ M\left ( NCS \right )_{6} \right ]^{\left ( -6+n \right )}](https://learn.careers360.com/latex-image/?%5Cleft%20%5B%20M%5Cleft%20%28%20NCS%20%5Cright%20%29_%7B6%7D%20%5Cright%20%5D%5E%7B%5Cleft%20%28%20-6+n%20%5Cright%20%29%7D)
![\left [ MF_{6} \right ]^{\left ( -6+n \right )}](https://learn.careers360.com/latex-image/?%5Cleft%20%5B%20MF_%7B6%7D%20%5Cright%20%5D%5E%7B%5Cleft%20%28%20-6+n%20%5Cright%20%29%7D)
![\left [ M \left ( NH _{3}\right )_{6}\right ]^{n+}](https://learn.careers360.com/latex-image/?%5Cleft%20%5B%20M%20%5Cleft%20%28%20NH%20_%7B3%7D%5Cright%20%29_%7B6%7D%5Cright%20%5D%5E%7Bn+%7D)
Option: 1 A-ii , B-i , C-iii
Option: 2 A-ii , B-iii , C-i
Option: 3 A-i , B-ii , C-iii
Option: 4 A-iii , B-i , C-ii
Answers (1)

(i) [M(NCS)](-6+n): NCS is a medium filed ligand. Thus, splitting will be required medium amount of energy. Thus will be medium.
(ii) [MF6](-6+n): F is a weak field ligand. Thus, splitting will be low and low energy will be required. Thus, will be highest.
(iii) [M(NH3)]n+: NH3 is strong filed ligand. Thus, splitting will be high and high energy will be required. Thus, will be lower.
Thus, the correct sequence is:
A-(iii), B-(i), C-(ii)
Therefore, Option(4) is correct.
JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
Similar Questions
-
5 g of Na2SO4 was dissolved in x g of H2O. The change in freezing point was found to be 3.820C. If Na2SO4 is 81.5% ionised, the value of x (K

A capacitor is made of two square plates each of side 'a' making a very small angle
-
A solution of m-chloroaniline, m-chlorophenol and m-chlorobenzoic acid in ethyl acetate was extracted initially with a saturated solution of NaHCO3 to give fraction A. The leftover organic phase was extracted with d


