Browse by Stream
-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


- Home
- Engineering
- The difference between the radii of 3rd and 4th orbits of is <img alt="\Delta R_{1}." src="http://entrancecorner.oncodecogs.com/gif.latex?%5CD
The difference between the radii of 3rd and 4th orbits of  is
is 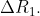 The difference between the radii of 3rd and 4th orbits of
The difference between the radii of 3rd and 4th orbits of  is
is 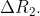 Ratio
Ratio 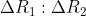 is :
is :
Option: 1 8 : 3
Option: 2 3 : 8
Option: 3 2 : 3
Option: 4 3 : 2
Answers (1)

We know the formula,
The radius of nth Bohr's Orbit
Where
n = principal quantum number of orbit.
Z = Atomic number
Now,
The difference between the radii of 3rd and 4th orbits of is
The difference between the radii of 3rd and 4th orbits of is
Ratio is
Therefore, the correct option is (3).
View full answer
JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE


