Browse by Stream
-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


- Home
- Engineering
- The number of geometrical isomers found in the metal complexes <img alt="\mathrm{\left[ PtCl _{2}\left( NH _{3}\right)_{2}\right],\left[ Ni ( CO )_{4}\right], \left[ Ru \left( H _{2} O \right)_{3} Cl _{3}\right] \text { and }\left[ CoCl _{2}\left( NH _
The number of geometrical isomers found in the metal complexes
![\mathrm{\left[ PtCl _{2}\left( NH _{3}\right)_{2}\right],\left[ Ni ( CO )_{4}\right], \left[ Ru \left( H _{2} O \right)_{3} Cl _{3}\right] \text { and }\left[ CoCl _{2}\left( NH _{3}\right)_{4}\right]^{+}}](https://learn.careers360.com/latex-image/?%5Cmathrm%7B%5Cleft%5B%20PtCl%20_%7B2%7D%5Cleft%28%20NH%20_%7B3%7D%5Cright%29_%7B2%7D%5Cright%5D%2C%5Cleft%5B%20Ni%20%28%20CO%20%29_%7B4%7D%5Cright%5D%2C%20%5Cleft%5B%20Ru%20%5Cleft%28%20H%20_%7B2%7D%20O%20%5Cright%29_%7B3%7D%20Cl%20_%7B3%7D%5Cright%5D%20%5Ctext%20%7B%20and%20%7D%5Cleft%5B%20CoCl%20_%7B2%7D%5Cleft%28%20NH%20_%7B3%7D%5Cright%29_%7B4%7D%5Cright%5D%5E%7B+%7D%7D) respectively, are :
respectively, are :
Option: 1 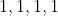
Option: 2 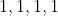
Option: 3 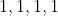
Option: 4 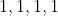
Option: 5 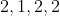
Option: 6 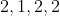
Option: 7 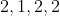
Option: 8 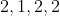
Option: 9 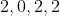
Option: 10 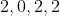
Option: 11 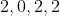
Option: 12 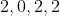
Option: 13 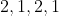
Option: 14 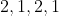
Option: 15 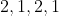
Option: 16 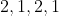
Answers (1)

The complexes along with their geometrical isomers are given below:
(a) : Square planar
(b) : tetrahedral complex.
Does not show Geometrical isomerism.
(c) : Octahedral
(d) : Octahedral
Hence, the correct answer is option (3)
View full answer
Similar Questions
-
5 g of Na2SO4 was dissolved in x g of H2O. The change in freezing point was found to be 3.820C. If Na2SO4 is 81.5% ionised, the value of x (K

A capacitor is made of two square plates each of side 'a' making a very small angle
-
A solution of m-chloroaniline, m-chlorophenol and m-chlorobenzoic acid in ethyl acetate was extracted initially with a saturated solution of NaHCO3 to give fraction A. The leftover organic phase was extracted with d




