-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Exams
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


All Questions
An ideal gas undergoes isothermal expansion at constant pressure. During the process :
Option: 1 enthalpy increases but entropy decreases.
Option: 2 enthalpy remains constant but entropy increases.
Option: 3 enthalpy decreases but entropy increases.
Option: 4 Both enthalpy and entropy remain constant.

The major product obtained in the following reaction is :
Option: 1 
Option: 2
Option: 3 
Option: 4 
The major product is represented by the option (D). DIBAL−H reduces esters to aldehydes. It also reduces carboxylic acids to aldehydes.

Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

A plane is inclined at an angle 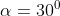 with respect to the horizontal. A particle is projected with a speed
with respect to the horizontal. A particle is projected with a speed 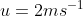 from the base of the plane , making an angle
from the base of the plane , making an angle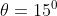 With respect to the plane as shown in the figure . The distance from the base ,at which the particle hits the plane is close to:
With respect to the plane as shown in the figure . The distance from the base ,at which the particle hits the plane is close to: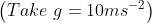

Option: 1 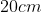
Option: 2 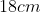
Option: 3 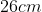
Option: 4 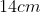
As we know on inclined plane the range is given by
The dimension of stopping potential in photoelectric effect in units of Planck's constant 'h', speed of light 'c' and Gravitational constant 'G' and ampere A is :
Option: 1
Option: 2
Option: 3
Option: 4
Let
Now,
So,
From here we will get: p-q=1---------(1)
2p+3q+r=2----------(2)
-p-2q-r=-3----------(3)
s=-1-----------(4)
From equation 1, 2,3 and 4 we will get: p=0,q=-1, r=5 and s=-1
So,
JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
Mischmetal is an alloy consisting mainly of :
Option: 1 lanthanoid metals
Option: 2 actionoid and transition metals
Option: 3 Lanthanoid and actionoid metals
Option: 4 actionoid metals
Mischmetal is an alloy of lanthanoid metals with Fe.
It consists of a lanthanoid metal (~95%) and iron (~5%) and traces of S, C, Ca, and Al.
Therefore, the correct option is (1).
View Full Answer(1)The incorrect statement(s) among (a)-(c) is (are):
(a) W(VI) is more stable than Cr(VI)
(b) in the presence of HCl, permaganate titrations provide satisfactory results.
(c) some lanthnoid oxides can be used as phosphorous
Option: 1 (b) and (c) only
Option: 2 (a) and (b) only
Option: 3 (b) only
Option: 4 (a) only
(a) W(VI) is more stable than Cr(VI). It is true and given in NCERT.
(b) in the presence of HCl permanganate titrations provide satisfactory results. It is incorrect.
Permanganate titrations in presence of hydrochloric acid are unsatisfactory since hydrochloric acid is oxidised to chlorine.
(c) Some individual Ln oxides are used as phosphors in television screens and similar fluorescing surfaces.
So,(b) is incorrect.
Therefore, the correct option is (3).
View Full Answer(1)NEET 2024 Most scoring concepts
- Just Study 32% of the NEET syllabus and Score up to 100% marks
The number of chiral carbons present in the molecule given below is __________.

Option: 1 5
Option: 2 5
Option: 3 -
Option: 4 -
Option: 5 -
Option: 6 -
Option: 7 -
Option: 8 -
Total chiral carbon = 5

View Full Answer(1)
Which one of the following graphs is not correct for ideal gas ?
 d = Density, P = Pressure, T = Temperature
d = Density, P = Pressure, T = Temperature
Option: 1 I
Option: 2 I
Option: 3 II
Option: 4 II
Option: 5 IV
Option: 6 IV
Option: 7 III
Option: 8 III
For ideal Gas

‘II’ Graph is incorrect.
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

If AB4 molecules is a polar molecule, a possible geometry of AB4 is :
Option: 1 Square pyramidal
Option: 2 Square pyramidal
Option: 3 Tetrahedral
Option: 4 Tetrahedral
Option: 5 Rectangular planar
Option: 6 Rectangular planar
Option: 7 Square planar
Option: 8 Square planar
(1) If AB4 molecule is a square pyramidal then it has one lone pair and their structure should be

and it should be polar because dipole moment of lone pair of 'A' never be cancelled by others.
(2) If AB4 molecule is a tetrahedral then it has no lone pair and their structure should be

and it should be non polar due to perfect symmetry.
(3) If AB4 molecule is a rectangular planar then

it should be non-polar because the vector sum of dipole moment is zero.
(4) If AB4 molecule is a square planar then

it should be non-polar because the vector sum of the dipole moment is zero.
View Full Answer(1)Which of the following is used for the preparation of colloids ?
Option: 1 Ostwald Process
Option: 2 Ostwald Process
Option: 3 Van Arkel Method
Option: 4 Van Arkel Method
Option: 5 Bredig's Arc Method
Option: 6 Bredig's Arc Method
Option: 7 Mond Process
Option: 8 Mond Process
Bredig's Arc method is used to form metal colloids.
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE


