-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Exams
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


All Questions
To find the standard potential of M3+/M electrode, the following cell is constituted: Pt/M/M3+(0.001 mol L−1)/Ag+(0.01 mol L−1)/Ag \ The emf of the cell is found to be 0.421 volt at 298 K. The standard potential of half reaction M3++3e− → M at 298 K will be : Given E Ag+/Ag at 298 K=0.80 Volte
Option: 1 0.38 Volt
Option: 2 0.32 Volt
Option: 3 1.28 Volt
Option: 4 0.66 Volt
The oxidation number of potassium in respectively is :
Option: 1
Option: 2
Option: 3
Option: 4
(In peroxide, the oxidation state of oxygen is -1)
(In superoxide, the oxidation state of oxygen is -1/2)
Therefore, Option(1) is correct.
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

Given that the standard potentials (Eo) of Cu2+|Cu and Cu+|Cu are 0.34V and 0.522V respectively, the Eo of Cu2+|Cu+ is:
Option: 1
Option: 2
Option: 3
Option: 4
1
View Full Answer(2)For an electrochemical cell the ratio
When this cell attains equilibrium is _____.
Option: 1
Option: 2 1.11
Option: 3 7.15
Option: 4 3.14
As we have learnt,
Nernst equation is given as
Now, the chemical reaction occuring in the cell is given as
Hence, the option number (1) is correct.
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
The equation that is incorrect is:
Option: 1
Option: 2
Option: 3
Option: 4
Both sides are not equal.
Therefore, Option(1) is correct.
View Full Answer(1)NEET 2024 Most scoring concepts
- Just Study 32% of the NEET syllabus and Score up to 100% marks
Potassium chlorate is prepared by the electrolysis of in basic solution
If only 60% of current utilized in the reaction, the time (hr) required to produce 10 g of
using a current of 2A is ___
The reaction occurs as follows:
Current efficiency = 60%
Now, moles of
Thus, moles of (assuming 100% efficiency)
Thus, actual moles of
Thus, t = 39387.76 sec. = 10.94 hours.
Thus, time required is approximately 11 hours.
View Full Answer(1)The redox reaction among the following is:
Option: 1 Formation of ozone from atmospheric oxygen in presence of sunlight
Option: 2 Redox of with
Option: 3 Combination of dinitrogen with dioxygen of 2000K.
Option: 4 Reaction of with

showing redox reaction, both oxidation and reduction.
Therefore, Option(3) is correct.
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

The oxidation states of iron atoms in compounds (A), (B) and (C), respectively, are x, y and z. The sum of x, y and z is______. 

View Full Answer(1)
For the given cell;  change in Gibbs energy
change in Gibbs energy 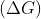 is negative, if :
is negative, if :
Option: 1 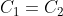
Option: 2 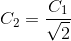
Option: 3 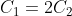
Option: 4 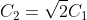
We know this formula

Only following the above condition.
Therefore, the correct option is (4).
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE


