-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Exams
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


All Questions
The concentration of fluoride, lead, nitrate, and iron in a water sample from an underground lake was found to be 1 ppm, 40 ppm, 100 ppm and 0.2 ppm, respectively. This water is unsuitable for drinking due to high concentration of :
Option: 1 Fluoride
Option: 10 Lead
Option: 19 Nitrate
Option: 36 Iron
As we learned in concept
Hard Water -
It contains calcium and Magnesium salt in the form of hydrogen carbonate , chloride and sulphate
- wherein
Hard water does not give Lathers with soap.
Maximum permissible concentration of constituents
lead : 50 ppm
nitrate : 50 ppm
Iron : 0.2 ppm
fluoride: 1 ppm
Therefore, option (3) is correct.
View Full Answer(1)Which one of the following statements about water is FALSE?
Option: 1 Water is oxidized to oxygen during photosynthesis..
Option: 2 Water can act both as an acid and as a base
Option: 3 There is extensive intramolecular hydrogen bonding in the condensed phase
Option: 4 Ice formed by heavy water sinks in normal water.
There is intermolecular hydrogen bounding in condensed phase of water and not intramolecular hydrogen bonding.
During Photosynthesis, Water is oxidised to Oxygen according to the reaction
Water can react with both acid as well as base
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

In comparison to the zeolite process for the removal of permanent hardness, the synthetic resins method is:
Option: 1 less efficient as the resins cannot be regenerated
Option: 2 less efficient as it exchange only anions
Option: 3More efficient as it can exchange only cations
Option: 4More efficient as it exchange both cations as well as anions
As we have learnt,
Therefore, Option(4) is correct.
View Full Answer(1)Hydrogen has three isotopes (A),(B), and (C). If the number of neutron(s) in (A), (B) and (C) respectively, are (x), (y) and (z), the sum of (x),(y) and (z) is :
Option: 1
Option: 2 3
Option: 3 2
Option: 4 1
As we have learnt,
Hydrogen has 3 isotopes. And these isotopes differ from each other because of presence of different number of neutrons.
- Protium,
- Deuterium,
or D
- Tritium,
or T
Number of neutrons in Protium is
Number of neutrons in Deuterium is
Number of neutrons in Tritium is
Thus, the correct answer is
Therefore, Option(2) is correct.
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
The equation that represents the water-gas shift reaction is :
Option: 1 ![\mathrm{CH_{4}(g)+H_{2}O(g)\xrightarrow[Ni]{1270K}CO(g)+3H_{2}(g)}](/latex-image/?%5Cmathrm%7BCH_%7B4%7D%28g%29+H_%7B2%7DO%28g%29%5Cxrightarrow%5BNi%5D%7B1270K%7DCO%28g%29+3H_%7B2%7D%28g%29%7D)
Option: 2 
Option: 3 
Option: 4 ![\mathrm{CO(g)+H_{2}O(g)\xrightarrow[catalyst]{673\; K}CO_{2}(g)+H_{2}(g)}](/latex-image/?%5Cmathrm%7BCO%28g%29+H_%7B2%7DO%28g%29%5Cxrightarrow%5Bcatalyst%5D%7B673%5C%3B%20K%7DCO_%7B2%7D%28g%29+H_%7B2%7D%28g%29%7D)
Let check given equations one by one-

Therefore, the correct option is (4).
View Full Answer(1)A 20.0 mL solution containing 0.2 g impure H2O2 reacts completely with 0.316 g of KMnO4 in acidic solution. The purity of H2O2 (in%) is _________ (mol.wt.of H2O2 =34, mol. wt. of KMnO4 = 158)
Option: 1 85
Option: 2 -
Option: 3 -
Option: 4 -
The reaction will be-
In above-balanced reaction
Electron transfer of H2O2 (n) = 2 (-2 to 0 of Oxygen)
Electron transfer of KmnO4 (n) = 5 (+7 to +2 of Mn)
We know this formula:
( Mole X n-factor ) of H2O2 = ( Mole X n-factor ) of KmnO4
Now
Mass = Mole X Molar mass
We know,
Ans = 85
View Full Answer(1)NEET 2024 Most scoring concepts
- Just Study 32% of the NEET syllabus and Score up to 100% marks
In the basic medium H2O2 exhibits which of the following reaction?

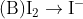
 Choose the most appropriate answer from the options given below:
Choose the most appropriate answer from the options given below:
Option: 1 A, B only
Option: 2 A only
Option: 3 B only
Option: 4 A, C only
In basic medium, oxidizing action of H2O2
In basic medium, reducing action of H2O2
In acidic medium, oxidising action of H2O2 , But not in a basic medium.
Therefore, the Correct option is (1)
View Full Answer(1)The INCORRECT statement (s) about heavy water is (are)
(A) used as a moderator in nuclear reactor
(B) obtained as a by-product in fertilizer industry
(C) used for the study of reaction mechanism
(D) has a higher dielectric constant than water
Choose the correct answer from the options given below
Option: 1 (C) only
Option: 2 (B) and (D) only
Option: 3 (D) only
Option: 4 (B) only
the correct statements about heavy water are
(A) used as a moderator in the nuclear reactor
(B) obtained as a by-product in the fertilizer industry
(C) used for the study of the reaction mechanism
But the incorrect statement about heavy water is
(D) has a higher dielectric constant than water.
because the dielectric constant of H2O is greater than heavy water.
Therefore, Correct option is (3)
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

Given below are two statements : Statement I : H2O2 can act as both oxidising and reducing agent in basic medium. Statement II : In the hydrogen economy, the energy is transmitted in the form of dihydrogen. In the light of above statments, choose the correct answer from the options given below :
Option: 1 Both statement I and II are true
Option: 2 Statement I is true and statement II is false
Option: 3 Both statement I and II are false
Option: 4 Statement I is false and but statement II is true
(a) H2O2 can act as oxidizing & reducing agent in both acidic & basic mediums.
(b) The basic principle of the hydrogen economy is the transportation and storage of energy in the form of liquids or gaseous dihydrogen. The advantage of hydrogen economy is that energy is transmitted in the form of dihydrogen and not as electric power.
View Full Answer(1)The correct statement about H2O2 are: A. used in the treatment of effluents. B. used as both oxidizing and reducing agents. C. the two hydroxyl groups lie in the same plane D. miscible with water Choose the correct answer from the options given below:
Option: 1 B,C and D only
Option: 2 A,B and D only
Option: 3 A, C and D only
Option: 4 A,B,C and D
(1) In H2O2 oxidation of oxygen is -1 Therefore acts both as oxidising and reducing agents.
(2) H2O2 is miscible in water due to intermolecular H-Bonding.
(3) H2O2 has open book structure in which both –OH groups are not on the same plane.

(4) H2O2 is used in the treatment of effluents.
So, correct statements about H2O2 are (A), (B) and (D) only.
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE


