-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Exams
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


All Questions
The value of is :
Option: 1
Option: 2
Option: 3
Option: 4
Trigonometric Identities -
Trigonometric Identities-
These identities are the equations that hold true regardless of the angle being chosen.
-
Allied Angles (Part 1) -
Allied Angles (Part 1)
Two angles or numbers are called allied iff their sum or difference is a multiple of π/2
-
sin (900 - θ) = cos (θ)
-
cos (900 - θ) = sin (θ)
-
tan (900 - θ) = cot (θ)
-
csc (900 - θ) = sec (θ)
-
sec (900 - θ) = csc (θ)
-
cot (900 - θ) = tan (θ)
-
sin (900 + θ) = cos (θ)
-
cos (900 + θ) = - sin (θ)
-
tan (900 + θ) = - cot (θ)
-
csc (900 + θ) = sec (θ)
-
sec (900 + θ) = - csc (θ)
-
cot (900 + θ) = - tan (θ)
-
The strength of an aqueous NaOH solution is most accurately determined by titrating:
(Note: consider that an appropriate indicator is used)
Option: 1 Aq.NaOH in a pipette and aqueous oxalic acid in a burette
Option: 2 Aq.NaOH in a volumetric flask and concentrated in a conical flask
Option: 3 Aq.NaOH in a burette and concentrated in a conical flask
Option: 4 Aq.NaOH in a burette and aqueous oxalic acid in a conical flask
As we have learnt,
To calculate the strength of NaOH, it is titrated against oxalic acid. For this purpose, NaOH is kept in a burette and oxalic acid in a conical flask.
Therefore, Option(4) is correct.
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

If and
then
is equal to ________.
Option: 1
Option: 2
Option: 3
Option: 4
Trigonometric Identities -
Trigonometric Identities-
These identities are the equations that hold true regardless of the angle being chosen.
-
Double Angle Formula and Reduction Formula -
Double Angle Formula and Reduction Formula
-
Correct Option (2)
View Full Answer(1)Reaction of an inorganic sulphite X with dilute 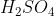 generates compound Y.Reaction of Y with
generates compound Y.Reaction of Y with 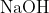 gives X. Further, the reaction of X with Y and water affords compound Z. Y and Z, respectively, are :
gives X. Further, the reaction of X with Y and water affords compound Z. Y and Z, respectively, are :
Option: 1 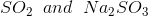
Option: 2 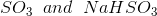
Option: 3 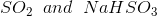
Option: 4 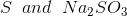
The reaction will be-

So, Y and Z, respectively, are
Therefore, the correct option is (3).
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
If you spill a chemical toilet cleaning liquid on your hand, your first aid would be:
Option: 1 aqueous NH3
Option: 2 gaseous NaHCO3
Option: 3 aqueous NaOH
Option: 4 Vinegar
Chemical toilet cleaning liquid contains acid and hence the first aid would be a weak base like NaHCO3.
Therefore, Option(2) is correct.
View Full Answer(1)Two poles, of length a metres and
of length
metres are erected at the same horizontal level with bases at
. If
and
, then :
Option: 1
Option: 2
Option: 3
Option: 4

NEET 2024 Most scoring concepts
- Just Study 32% of the NEET syllabus and Score up to 100% marks
The addition of dilute  salt solution will give :
salt solution will give :
Option: 1 Precipitate of 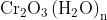
Option: 2 Precipitate of 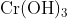
Option: 3 Precipitate of ![\left[\mathrm{Cr}(\mathrm{OH})_{6}\right]^{3-}](https://learn.careers360.com/latex-image/?%5Cleft%5B%5Cmathrm%7BCr%7D%28%5Cmathrm%7BOH%7D%29_%7B6%7D%5Cright%5D%5E%7B3-%7D)
Option: 4 A solution of ![\left[\mathrm{Cr}(\mathrm{OH})_{4}\right]^{-}](https://learn.careers360.com/latex-image/?%5Cleft%5B%5Cmathrm%7BCr%7D%28%5Cmathrm%7BOH%7D%29_%7B4%7D%5Cright%5D%5E%7B-%7D)
Addition of dilute to a salt solution of
will lead to the formation of a precipitate of hydrated chromium (III) oxide.
Hence, the correct answer is option (1)
View Full Answer(1)Acidic ferric chloride solution on treatment with excess of potassium ferrocyanide gives a Prussian blue coloured colloidal species. It is :
Option: 1 ![\mathrm{HFe}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]](https://learn.careers360.com/latex-image/?%5Cmathrm%7BHFe%7D%5Cleft%5B%5Cmathrm%7BFe%7D%28%5Cmathrm%7BCN%7D%29_%7B6%7D%5Cright%5D)
Option: 2 ![\mathrm{K}_{5} \mathrm{Fe}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]_{2}](https://learn.careers360.com/latex-image/?%5Cmathrm%7BK%7D_%7B5%7D%20%5Cmathrm%7BFe%7D%5Cleft%5B%5Cmathrm%7BFe%7D%28%5Cmathrm%7BCN%7D%29_%7B6%7D%5Cright%5D_%7B2%7D)
Option: 3 ![\mathrm{Fe}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]_{3}](https://learn.careers360.com/latex-image/?%5Cmathrm%7BFe%7D_%7B4%7D%5Cleft%5B%5Cmathrm%7BFe%7D%28%5Cmathrm%7BCN%7D%29_%7B6%7D%5Cright%5D_%7B3%7D)
Option: 4 ![\mathrm{KFe}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]](https://learn.careers360.com/latex-image/?%5Cmathrm%7BKFe%7D%5Cleft%5B%5Cmathrm%7BFe%7D%28%5Cmathrm%7BCN%7D%29_%7B6%7D%5Cright%5D)
ions react with excess of potassium ferrocyanide to form
.
The reaction is given below:
Hence,the correct answer is Option (4)
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

Match List - I with List - II :
| List-I (Metal Ion) | List-II (Group in Qualitative analysis) |
| (i) Group - III | |
| (ii) Group - IIA | |
| (iii) Group - IV | |
| (iv) Group - IIB |
Option: 1
Option: 2
Option: 3
Option: 4
The correct match of basic radicalsand their groups in Qualitative analysis is given below
Hence the correct answer is option (1)
View Full Answer(1)Consider the sulphides and
. Number of these sulphides soluble in
is_________.
The sulphide which are soluble in are
Sulphides insoluble in are
and
Hence, the correct answer is 4
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE


