Browse by Stream
-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Top Schools
Products & Resources
-
Study Abroad
Top Countries
Resources
-
Arts, Commerce & Sciences
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Exams
Colleges & Courses
Predictors
-
Learn
Law Preparation
MBA Preparation
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Exams
Predictors & Articles
Colleges
Resources
-
Media, Mass Communication and Journalism
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


- Home
- Engineering
- salt reacts with potassium iodide to giveOption: 1 <
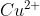 salt reacts with potassium iodide to give
salt reacts with potassium iodide to give
Option: 1 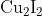
Option: 2 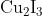
Option: 3 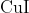
Option: 4 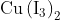
Answers (1)

reacts with
to form precipitate of
.
is reduced to
while Iodide ions are oxidised to Iodine as shown below
The complexes with
to give tri iodide ions
which forms a reddish solution.
Hence, the correct answer is option (1).
View full answer
JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
Similar Questions
-
5 g of Na2SO4 was dissolved in x g of H2O. The change in freezing point was found to be 3.820C. If Na2SO4 is 81.5% ionised, the value of x (K

A capacitor is made of two square plates each of side 'a' making a very small angle
-
A solution of m-chloroaniline, m-chlorophenol and m-chlorobenzoic acid in ethyl acetate was extracted initially with a saturated solution of NaHCO3 to give fraction A. The leftover organic phase was extracted with d


